Diseases such as age-related macular degeneration and diabetic retinopathy that affect the posterior eye segment, particularly the retina, are the leading cause of blindness worldwide. While the eye is readily accessible from the outside of the body, conventional eye drops are unable to deliver sufficient drug concentrations to the back of the eye due to the various barriers in place to protect it from the environment. To overcome these limitations, drug solutions are generally injected directly into the eyeball to treat retinal diseases, a procedure that has to be repeated frequently, is very unpleasant and may result in complications.
 With over 18 million intravitreal injections performed annually, a massive burden for both healthcare professionals and patients arises due to the frequency of the injections required. Thus, technologies that can reduce the treatment burden are of high demand. To date, four sustained-release intraocular implants targeting back-of-the-eye conditions have been marketed; however, some of these require surgical implantation and removal after the drug has been released.
With over 18 million intravitreal injections performed annually, a massive burden for both healthcare professionals and patients arises due to the frequency of the injections required. Thus, technologies that can reduce the treatment burden are of high demand. To date, four sustained-release intraocular implants targeting back-of-the-eye conditions have been marketed; however, some of these require surgical implantation and removal after the drug has been released.
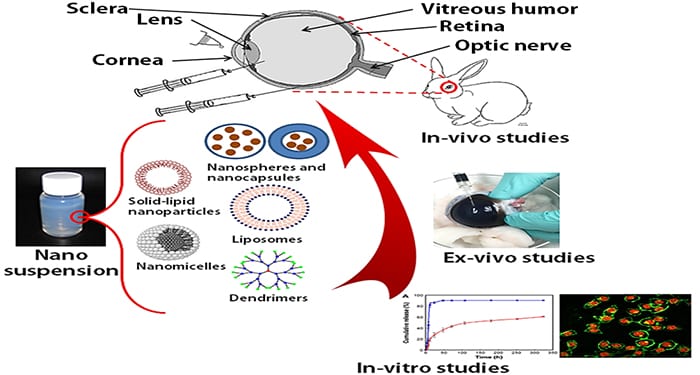
A recent review article published in WIREs Nanomedicine and Nanobiotechnology by Rohit Bisht and colleagues from the University of Auckland, New Zealand, and the University of Missouri-Kansas City, USA, reports on recent developments and preclinical studies in the area of nanotechnology-based systems for minimally invasive and effective long-term treatment of retinal diseases. The biodegradable nature and small size of such nanocarriers hereby avoids the need for surgical implantation and removal, while the sustained drug release characteristics reduce the need for frequent intravitreal injections. Moreover, the highly modifiable surface properties allow specific targeting of these systems to the diseased tissues.
Various studies have established the potential of nanostructured carriers, such as polymeric nanoparticles, nanostructured lipid carriers, liposomes, polymeric micelles and dendrimers, to maintain therapeutic drug concentrations over prolonged time periods. In addition, well-established preparation methods, formulation stability and biocompatibility render them suitable candidates to be transformed into successfully marketed formulations. With recent developments and successful clinical trials, nanocarriers may radically alter the way many retinal blinding diseases are currently treated with the next decade promising great strides to be seen with regards to safe and effective retinal targeted therapy.
Contributed by the Authors.

















