For millions of patients with cancer, chemotherapy is their best chance for recovery. However, this treatment is beset with problems of efficiency and brutal side effects.
To improve on these challenges, scientists have developed nano-sized particles called nanovehicles, which facilitate the safe delivery of anti-cancer drugs directly to cells. Now, the next stage in the evolution of nanovehicle drug deliver relies on unique group of molecules called pillararenes, which ensure that drugs are released to tumor cells only, sparing the healthy tissue and minimizing side effects.
“The key to improving tumor therapy effectiveness is to design drug delivery platforms that can precisely locate tumors and control drug release,” said Luzhi Liu, professor at Yulin Normal University in China and author of a recent study detailing the use of pillararenes to enhance the capabilities of nanovehicle drug delivery.
Liu believes that pillararene-coated nanovehicles can also address another common problem with chemotherapy: drug resistance. This is conventionally done using multiple methods to attack tumors — killing rogue cells with toxic substances is one, or starving tumors of vital resources. Therefore, nanovehicles need to carry multiple drugs and release them on-demand.
Due to their molecular structure, pillararene help nanovehicles check all these boxes, allowing them to expand their payload and control precisely when and where the drugs are released.
Controllable valves for drug release
This is all possible thanks to pillararenes symmetrical, pillar-like structure, which contains a cavity in which other molecules can sit. They also excel at forming reversible, non-covalent bonds with other molecules, meaning they bond using forces like electrostatics, ionic bonding, or others that don’t involve permanently sharing electrons.
These properties make it an excellent candidate for creating nanovalves. “Nanovalves are like a cap or a door lock that covers the surface of the nanovehicles,” explained Liu. “They generally consist of larger molecules that block the pathway for the drug to go out in order to control the release of the drug.” Coating the vehicle in pillararene nanovalves can improve their drug-carrying capacity, but also control the rate of drug release, added Liu.
Liu and his team modified conventional gold nanoparticles, covering them with pillararene nanovalves that were loaded with two types of treatments: one chemotherapy and one starvation therapy.
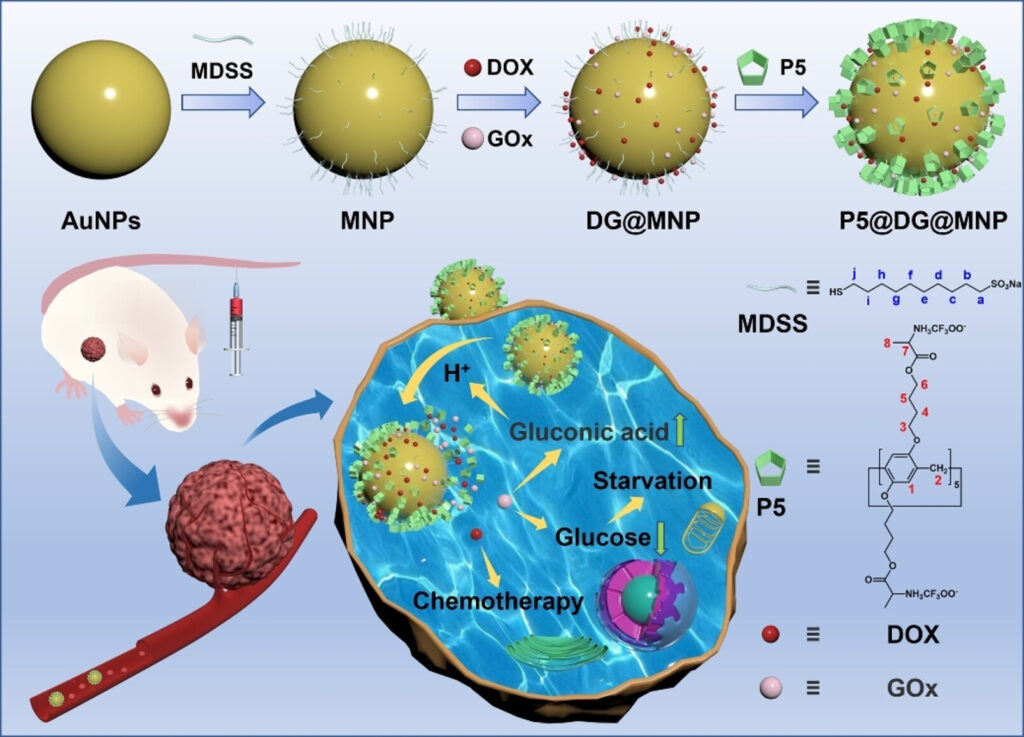
The valves were designed in two different ways to allow the controlled and separate release of this combination therapy. Both release mechanisms work by taking advantage of non-covalent bonding between the nanovalves and other molecules the researchers added to the surface.
In this case, these molecules are sulfonates, chosen because they bond to the nanovalves, keeping them closed, but can release them in response to pH or heat. The environment around a tumor is slightly acidic, and, in these conditions, an electrostatic interaction occurs between the sulfonate molecules and the pillararene, weakening their attraction to each other, opening the nanovalve.
A similar reaction occurs between pillararene and sulfonic acid groups when the temperature increases. Using focused light to precisely heat up the area of a known tumor would, along with the acidic tumor environment, trigger release of the drugs only where they are needed.
Increasing the chemotherapy payload
In tests on tumor cells cultured in the lab as well as mice, the new nanovehicles performed as designed.
“As we expected, both […] experiments demonstrated that this nanoplatform has good biocompatibility, and this multi-functional nanoplatform for combination therapy showed superior anticancer effects compared to chemotherapy alone,” said Liu.
The next step for the group is to add more types of treatments to the nanovehicles. “We plan to integrate more therapeutic approaches, such as photothermal and photodynamic therapies, into the nanodrug delivery system to further improve the efficiency of tumor treatment,” Liu said.
The results are promising and the mice receiving treatment did not lose weight, an indicator that it was well tolerated. Trials in human patients will ultimately determine how effective this treatment is and what, if any, side effects remain, but there are still many hurdles to overcome.
Reference: Jianfeng Jian, et al. A Nanoplatform Based on Pillar[5]arene Nanovalves for Combined Drug Delivery and Enhanced Antitumor Activity, Chemistry – A European Journal (2024). DOI: 10.1002/chem.202400007
Feature image credit: The Halal Design Studio on Unsplash