2-hydroxyethyl methacrylate (HEMA)-based polymers find a wide range of applications, particularly in the biomedical field, such as for contact lens materials, coatings and hydrogels. Historically, such polymers were made by conventional radical polymerization, which does not permit the tight control of molecular weight and does not maintain the requisite chain end functionality to access more sophisticated microstructures that is essential for improved products in the sectors mentioned above.
2-hydroxyethyl methacrylate (HEMA)-based polymers find a wide range of applications, particularly in the biomedical field, such as for contact lens materials, coatings and hydrogels. Historically, such polymers were made by conventional radical polymerization, which does not permit the tight control of molecular weight and does not maintain the requisite chain end functionality to access more sophisticated microstructures that is essential for improved products in the sectors mentioned above.
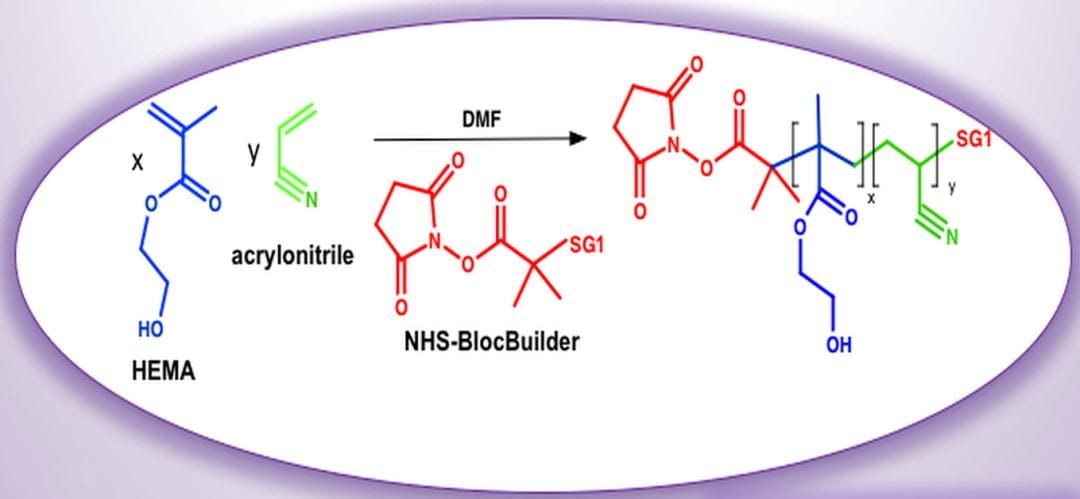
For example, narrower molecular weight distributions make coatings less viscous and thus easier to apply and allow for less solvent to be used (lower volatile organic content). Further, additional functionality can be built into a single polymer chain via block copolymerization, which is often required of current biomedical materials. HEMA has been polymerized by other reversible deactivation radical polymerization (RDRP) techniques such as atom transfer radical polymerization (ATRP) and reversible activation fragmentation transfer polymerization (RAFT), where residues from the polymerization process may not be desirable and require additional treatment. Up to now, HEMA-rich resins were not accessed by nitroxide mediated polymerization (NMP), which is an interesting alternative to the other RDRP processes, as it does not require extensive post-polymerization treatment.
In their recent article, W. Wei and M. Maric (McGill University, Montreal) explored the reversible deactivation radical polymerization of 2-hydroxyethyl methacrylate using nitroxide mediated polymerization. Here the controlled polymerization of HEMA using a low concentration of »5 mol% of controlling co-monomer such as styrene or acrylonitrile, is applied using a commercially available alkoxyamine. Other NMP systems using acrylonitrile as controlling co-monomer were found to exhibit non-cytotoxic behavior and thus the HEMA resins produced are promising for biomedical applications.