Biomaterials placed in the body, such as hernia meshes, joint replacements, heart valves, and artery stents, are often treated with proteins such as collagen prior to implantation. To ensure the proteins are strongly attached, the biomaterials are typically chemically treated first. Traditional methods of measuring the effectiveness of surface chemical treatments involve significant error and conjecture. Proteins that improve cell attachment have specific pKa’s and optimum binding requirements that may differ from the conditions required for chemical labeling.
Using Collagen ELISA as a Method of Surface Chemical Analysis
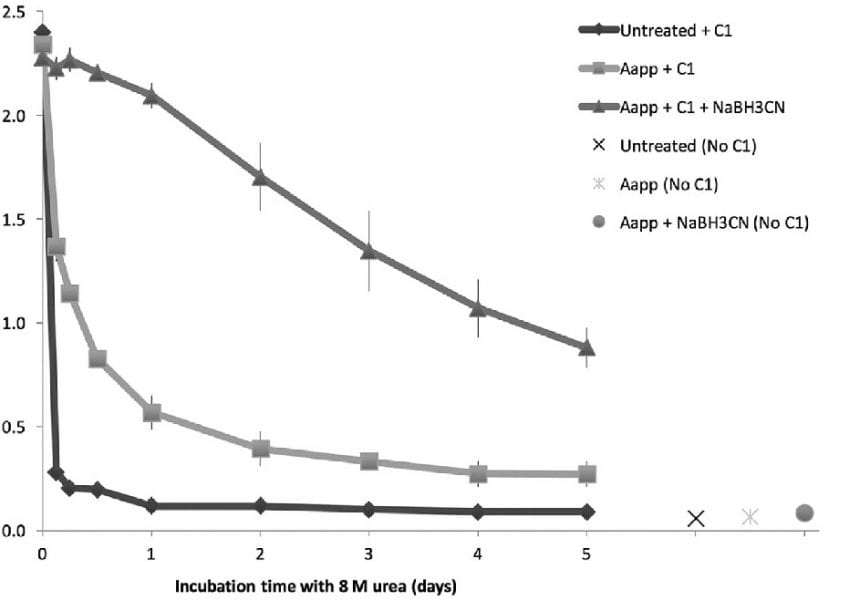
In a recently published article that will appear in Plasma Processes and Polymers researches of the Barwon Biomedical Research of University Hospital Geelong, the St. Vincent’s Hospital Melbourne and the Institute for Frontier Materials of the Deakin University in Australia have researched a new technique of surface chemical analysis: measuring residual collagen after stressing its attachment with concentrated urea appeared to suffice for optimizing surface chemical treatment parameters.
The utility of collagen ELISA to optimize acetaldehyde glow discharge polymerization reactor parameters was tested. Accurate stepwise increases in collagen conjugation strength were demonstrated by incubating specimens in 8 M urea for 5–8 days followed by ELISA to test for residual surface collagen. Surface modifications also were assessed by XPS. The results indicated that ELISA after bond-stressing with urea may suffice for optimizing surface fictionalization. This research demonstrates the utility of this technique for optimizing glow discharge polymerization parameters, demonstrating protein function and covalent bonding. This technique also suggested that in many situations where protein attachment is the aim, traditional methods of surface chemical analysis may be superfluous.