Nitric oxide (NO)–a reactive free radical–is a unique type of transmitter in the nervous system. Once produced, NO diffuses rapidly across membranes and has a half-life of <30 s.
It acts on neighboring cells and can affect processes more than 100 µm away. NO plays a central role in the physiology and the pathophysiology of many systems in human organs, acting as a messenger in many diverse functions.
Sufficient levels of NO production are necessary in protecting an organ; however, sustained NO levels result in direct tissue toxicity, and chronic expression of NO is associated with various carcinomas and inflammatory conditions. Due to its multifaceted role, NO must be administered at a right dose and at a specific site.
Tao Yang, Alexander N. Zelikin, and Rona Chandrawati from UNSW Sydney and Aarhus University give a comprehensive review of the current landscape of NO-releasing platforms, with emphasis on their designs, syntheses, NO flux, and NO release half-life. They summarize the advantages and drawbacks of NO‐releasing platforms, and highlight recent advancements in nanotechnology and drug delivery are highlighted. The challenges of working with a free-radical molecule with a short half-life, and how this translates to therapeutic applications, are addressed. Various combinations of NO donors and biomaterials (e.g., liposomes, polymeric carriers, and MOFs) are also discussed.
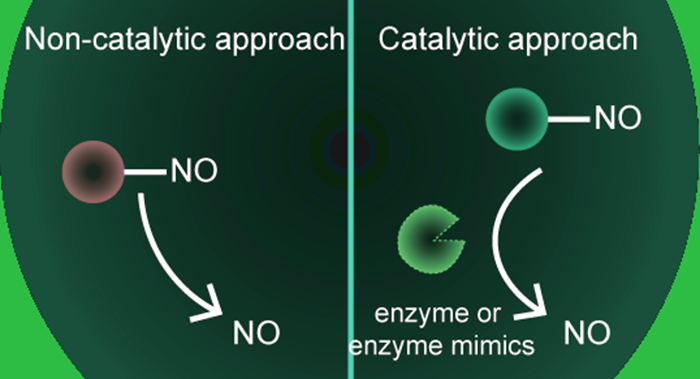
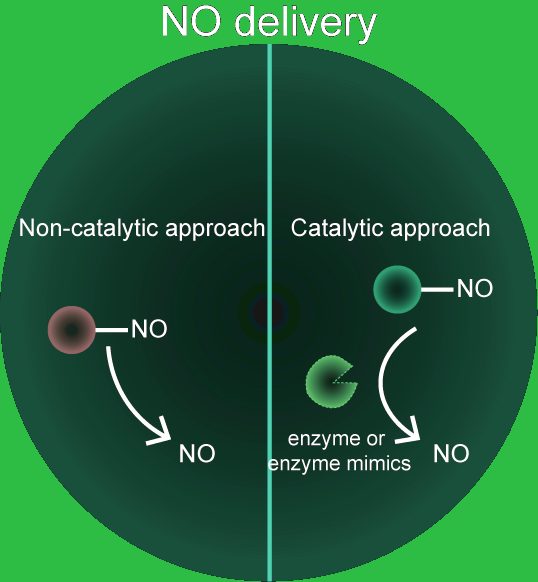
A brief overview if given of the importance of NO as a multifaceted biomolecule involved in multiple biological processes. The need for better engineered NO-releasing biomaterials is described, with an emphasis on a localized, controlled, and site-specific NO-delivery platform to maximize therapeutic efficacy and reduce off-target effects. The advantages and drawbacks of both non-catalytic and catalytic NO-delivery platforms, and the potential translation of these technologies is also discussed.