High mobility conjugated polymers have attracted great attention because of their applications in printable electronics. Charge transport capability of semiconducting conjugated polymers is closely related to the π-π interaction between conjugated backbones. One of the approaches to enhance the π-π interaction of conjugated polymers is to incorporate large heteroacene units into the polymer backbones to enhance the coplanarity and thus the intermolecular interaction. However, incorporation of large fused aromatics into conjugated polymers often causes a dramatic decrease in solubility and self-assembly ability.
High mobility conjugated polymers have attracted great attention because of their applications in printable electronics. Charge transport capability of semiconducting conjugated polymers is closely related to the π-π interaction between conjugated backbones. One of the approaches to enhance the π-π interaction of conjugated polymers is to incorporate large heteroacene units into the polymer backbones to enhance the coplanarity and thus the intermolecular interaction. However, incorporation of large fused aromatics into conjugated polymers often causes a dramatic decrease in solubility and self-assembly ability.
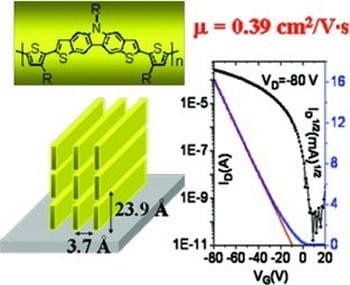
Yanhou Geng (Changchun Institute of Applied Chemistry) and co-workers have now synthesized a new heteroacene comprising five fused rings, i.e. dithieno[3,2-b:6,7-b]carbazole, for constructing high mobility conjugated polymers. In this new heteroacene unit, an alkyl chain can be introduced at the N-atom for improving the solubility and self-assembly ability of the resulting conjugated polymers. In thin films, the polymer backbones dominantly adopt an edge-on orientation with respect to the substrate to form highly ordered thin films with a lamellar spacing of ~24 Å and a π-stacking distance of 3.7 Å. A remarkably high hole-mobility of up to 0.39 cm2V-1s-1 has been demonstrated as measured in bottom gate and top-contact organic thin-film transistors.
This mobility value is the highest among conjugated polymers containing heteroacenes with more than four fused rings rendering the material a promising candidate for organic thin film transistors.