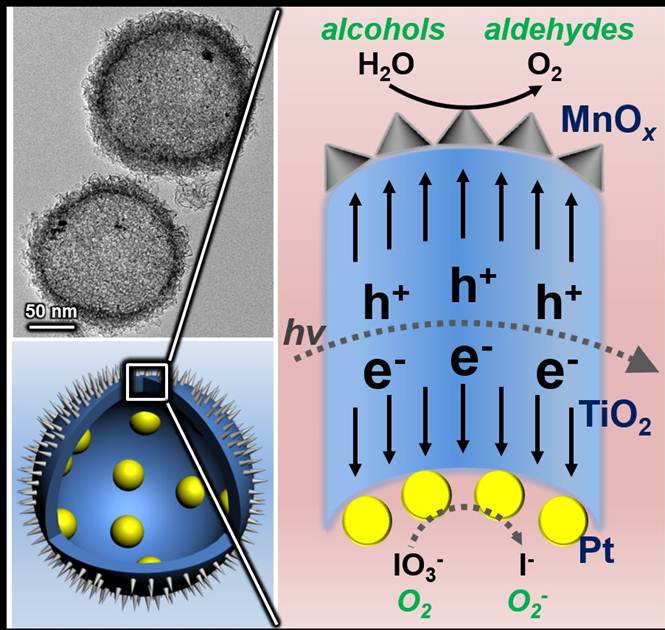
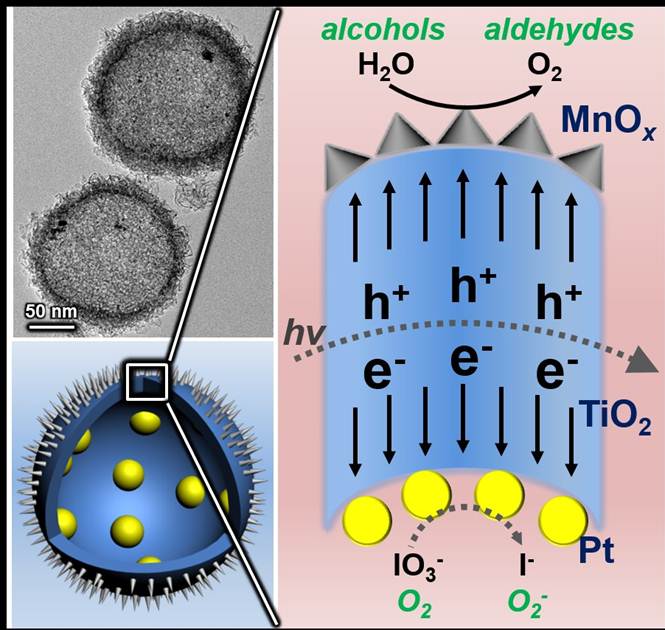
Solar energy conversion of semiconductor photocatalysts requires efficient charge separation, which is the bottleneck to promote the light-driven water splitting and other photocatalytic oxidation processes. The use of cocatalysts has been proved to be an effective approach to accelerate the charge separation and enhance the efficiency of light-assisted process. However, in previous reported cocatalysts, the close sites between the reduction and oxidation catalysts may easily lead to electron-hole recombination, and thus are unfavorable for the redox reaction.
Prof. Jinlong Gong and his colleagues at Tianjin University have recently developed a cocatalyst with complete spatial separation of oxidation and reduction sites. In this novel catalyst, different cocatalysts are separated by TiO2 shells completely. Upon generation from TiO2, electrons and holes will flow inward and outward of the spherical TiO2 shells, respectively, accumulating on corresponding cocatalysts, and then take part in redox reactions. In addition, light will pass through thin TiO2 shells and keep reflecting in the cavity, increasing the scattering length for enhanced light-absorption. Using this cocatalyst, remarkably efficient oxidation of water and benzyl alcohol were demonstrated, confirming the success of this novel photocatalytic system.
The mechanism of the current cocatalyst may inspire the design of similar catalysts with high activity, which will benefit the light-driven water splitting and other relevant organic synthesis of fine chemicals.