The fabrication of materials that mimic native tissue or bone—the underlying goal of tissue engineering—has become the aim of many researchers in recent decades. Ideally, tissue mimetic materials could be used for creating physiologically relevant models for drug screening or cosmetic testing, for functional grafts for wounds, or perhaps even for the creation of whole organs.
However, the tissues and bones that make up our bodies are complex and formed from a variety of components. This means that replicating these materials is no simple feat.
One targeted challenge in tissue engineering is the osteochondral interface—the region that exists between cartilage and bone. This transition from cartilage to bone can be viewed as an inhomogeneous construct with a natural gradient. Complex inhomogeneous tissues like this are found in many areas of the body, like tendons and the central nervous system.
When fabricating gradient materials for tissue engineering, techniques such as multi-channel microfluidics or 3D printing have been used in the past; however, these approaches are limited by the use of specialist apparatus or specific material parameters. While gradients have been achieved through photopatterning or magnetic field alignment, these methods can introduce even more specific material requirements.
Chunching Li, James P. K. Armstrong, Molly M. Stevens and their colleagues at Imperial College London have developed a simple, quick and versatile way of creating gradients across multiple materials. By a single injection of one liquid into a second denser liquid, they were able to produce tunable gradients by capitalizing on the fundamental force of buoyancy.
These liquid gradients could then be further preserved by gelation or polymerization. Beyond just material gradients, the team investigated using this technique to form gradients with various “cargos” like nanoparticles or small molecules. They were even able to tune sharp and gradient transitions in the material.
Interestingly, they found that by uniformly introducing growth factors, which promote certain cells to begin a process known as osteogenesis—or bone formation—to a gradient structure, they were able to form a construct with distinct bone and cartilage zones.
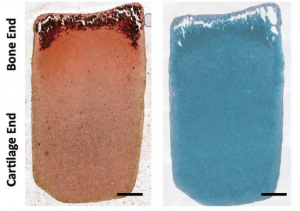
Despite an initially homogeneous distribution of cells and a smooth material gradient, after culturing for 28 days, the mature construct was comprised of a sharp gradient resembling the hallmark “tidemark” zone of the native osteochondral interface.
This versatile, quick and easy-to-use technique opens up possibilities for a plethora of applications in gradient material fabrication and interfacial tissue engineering.

















