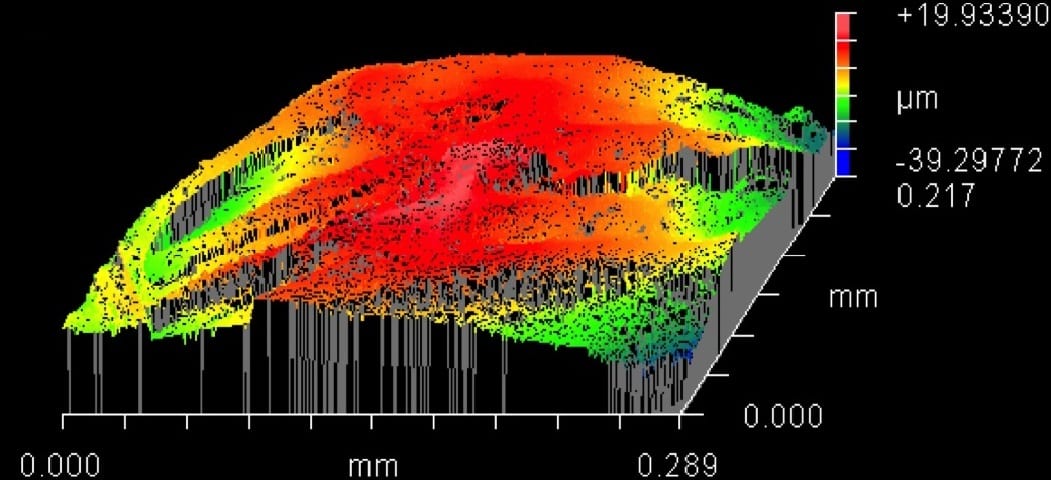
Articular cartilage, the thin tissue that lines the ends of long bones, is a highly complex biomaterial with a very low coefficient of friction. This allows the tissue to withstand millions of cycles of joint-loading over decades of wear. Once damaged, mechanical function is lost, and the progressive degeneration of the tissue induces significant pain and disability.
Efforts to repair and regenerate cartilage, to treat injury and prevent osteoarthritis, have often included the use of hydrogels. The main obstacles met in these studies are related to matching the characteristics and distinct abilities of the natural tissue. Now, a research group from the Duke University, in Durham, North Carolina, have developed a three-dimensionally woven fiber scaffold which, once infused with a hydrogel, mimics the load-bearing and tribological properties of native cartilage.
The woven fiber scaffold provides controlled tensile and compressive mechanical properties in three dimensions. In this work, this scaffold is infiltrated with an interpenetrating network hydrogel. The infusion of the hydrogel (consisting of an interpenetrating network of alginate and polyacrylamide) provides the scaffold with high toughness and a low coeffecient of friction. This biomimetic material structure is a versatile fiber-reinforced composite, and a potential acellular or cell-based replacement for cartilage.