 Ionic liquids (IL) are salts with a melting temperature below the boiling point of water (100 °C). When an IL molecule contains a polymerizable unit, such as a vinyl group, it can be polymerized to form chain-like macromolecules, that is, poly(ionic liquid)s (PILs). Vinylimidazolium-type ILs can be readily polymerized.
Ionic liquids (IL) are salts with a melting temperature below the boiling point of water (100 °C). When an IL molecule contains a polymerizable unit, such as a vinyl group, it can be polymerized to form chain-like macromolecules, that is, poly(ionic liquid)s (PILs). Vinylimidazolium-type ILs can be readily polymerized.
When these monomers carry long alkyl chains (C12-C18), the polymerization produces a stable colloidal dispersion of PIL nanoparticles, which exhibit distinctive and highly ordered inner structures. The PIL nanoparticles can be crosslinked to improve its stability against aggressive organic solvents. However, a question yet not answered is: what is the internal structure of these crosslinked nanoparticles?
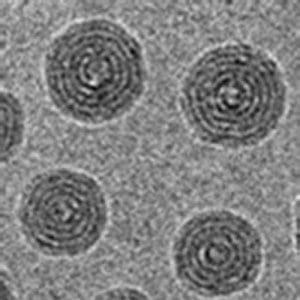
Researchers from the Max Planck Institute of Colloids and Interfaces in Golm (Germany) have now made use of cryogenic transmission electron microscopy (Cryo-TEM) to characterize the crosslinked PIL nanoparticles. Nanoparticles of 20-40 nm in size were prepared via precipitation polymerization of vinylimidazolium type ionic liquid monomers with long alkyl tails (C12-C18) in the presence of a divinylimidazolium crosslinking agent. Cryo-TEM revealed that the crosslinked PIL nanoparticles resembled centric multilamellar vesicles of different geometry, depending on the exact length of the quaternizing alkyl chains. The nanoparticle size was found to expand when produced from polymerizations at higher concentration of the monomer, crosslinking agent and externally added salt, and by the replacement of Br by dicyanamide anion. Such size expansion of the nanoparticles can be either isotropic or anisotropic via their linear attachment.
This study provides new insights into the formation mechanism of such highly ordered centric multilamellar vesicles from ionic liquid monomers. The study on the size controlling effect helps to prepare a homologous series of colloidal templates for materials application.