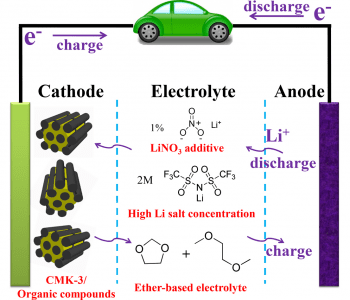
The use of organic materials as cathodes in organic lithium-ion batteries is attractive due to their high theoretical capacity, controllable design at the molecular level, and the sustainability of the required resources. However, their use as cathodic materials suffers some drawbacks, such as their low conductivity, which leads to poor rate performance, and their high solubility in traditional, carbonate-based electrolytes. To overcome these drawbacks, and progress towards high-performance organic cathodes, researchers at Nankai University, China have demonstrated optimized electrolytes and cathode configurations to maintain high capacity of the organic electrodes, while increasing stability, cycling and rate performance. Taking inspiration from Li–S batteries, which suffer similar problems, the issues of solubility and low conductivity were overcome by the use of ether-based electrolytes with additives and a mesoporous carbon-based framework for a nanocomposite electrode.
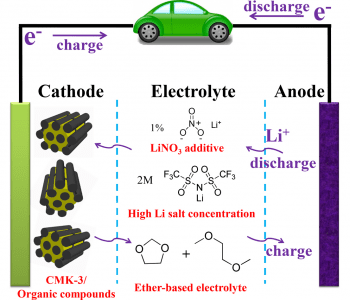 Firstly, the concentration of the ether-based electrolyte is optimized. Lithium bis(trifluoromethanesulfonyl)imide salt was mixed with 1,3-dioxolane/dimethoxyethane solvent at concentrations of 1, 2, 3, and 4 m (1, 2, 3, 4 M-DD). Using cells with a lithium metal anode and organic 9,10-anthraquinone (AQ) cathode, the performance of these electrolytes was compared with that of a traditional LiPF6 in diethyl carbonate electrolyte. As the salt concentrations increase, discharge capacity and ionic conductivity at room temperature was found to gradually decrease. On the other hand, the solubility of the organic electrode was greatly improved in the ether-based electrolytes compared to the traditional electrolyte. Based on the ionic conductivity, discharge capacity, and cycling performance, 2m-DD was chosen as the optimal electrolyte.
Firstly, the concentration of the ether-based electrolyte is optimized. Lithium bis(trifluoromethanesulfonyl)imide salt was mixed with 1,3-dioxolane/dimethoxyethane solvent at concentrations of 1, 2, 3, and 4 m (1, 2, 3, 4 M-DD). Using cells with a lithium metal anode and organic 9,10-anthraquinone (AQ) cathode, the performance of these electrolytes was compared with that of a traditional LiPF6 in diethyl carbonate electrolyte. As the salt concentrations increase, discharge capacity and ionic conductivity at room temperature was found to gradually decrease. On the other hand, the solubility of the organic electrode was greatly improved in the ether-based electrolytes compared to the traditional electrolyte. Based on the ionic conductivity, discharge capacity, and cycling performance, 2m-DD was chosen as the optimal electrolyte.
In the Li–S batteries, a LiNO4 additive is found to form a protective film over the anode, and increase cycling performance. The concentration of additive was also optimized, after testing concentrations of 0.5%, 1%, and 2%. As the concentration increases, the capacity decreases, but the protective effect on the anode increases the cycling performance, with 94% capacity retention achieved after 20 cycles at 1% concentration, compared to 66% for the additive-free 2M-DD. The surface of the anode after cycling was found to be smooth, confirming the presence of the protective film. The optimal additive concentration was determined to be 1%, such that the protective film is formed on the anode, but ion transfer is not hindered.
Finally, the composition of the nanocomposite cathode is also optimized, since the pure AQ has low electrical conductivity. Organic AQ is impregnated into mesoporous carbon at varying ratios of 18.1 wt%, 30.0 wt%, and 46.3 wt% carbon, to form composite AQC electrodes. High carbon content significantly improves the performance at high currents, due to the lowered ion transfer impedance and nanoconfinement of the AC in the porous carbon. The 30 wt% ratio was found to give the best overall performance.
The fully optimized cell, with Li anode, 30 wt% carbon AQC cathode, and 1% LiNO4 2M-DD electrolyte demonstrated an excellent capacity retention of 85% after 100 cycles, much higher than previously reported cells with organic cathodes. This strategy has also been proven to be applicable to other biphenyl quinone-based cathode materials, achieving over 97% of theoretical capacity and up to 84% capacity retention after 100 cycles, a significant advancement in the development of high performance organic lithium batteries.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.