Many sustainable technologies rely on the ability to generate oxygen molecules, either in alkaline environments, such as in metal–air batteries, or in neutral/acidic environments, as in water splitting reactions. These reactions, known as oxygen evolution reactions (OER), generally proceed slowly, by electrolysis, requiring the use of catalysts deposited onto electrodes to increase rate and yield.
Performance of the electrocatalysts can be improved by modifying the chemical composition of the (typically metal-oxide based) catalysts, or by changing the structure of the supporting electrode to increase the surface area over which the OER reaction can take place. Unfortunately, even for catalysts with optimized chemical compositions, the weak interactions between the catalyst and the electrode material (often glassy carbon or titanium foils) means that the lifetime of the electrode–catalyst system is limited, as the catalyst is not well bonded to the surface of the electrode. This leads to substantial reduction in performance over time.
Consequently, development of an electrocatalysis system that does not degrade significantly would be beneficial for technologies that generate oxygen via the OER, as well as other reactions that rely on similar catalysts, including hydrogen evolution reactions, oxygen reduction reactions and carbon monoxide oxidation.
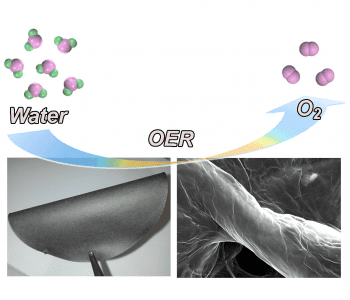 Researchers at the University of Adelaide have now developed a novel electrocatalyst system consisting of graphene and carbon nitrate films assembled on the surface of cellulous fiber paper. There is strong bonding between the graphene oxide–carbon nitrate film and the functional groups of cellulose paper, so loss of the catalyst from the supporting electrode is not an issue. The electrocatalyst films are prepared by merging graphene oxide and carbon nitrate nanosheets in dispersion, into which the cellulose fiber paper is dipped.
Researchers at the University of Adelaide have now developed a novel electrocatalyst system consisting of graphene and carbon nitrate films assembled on the surface of cellulous fiber paper. There is strong bonding between the graphene oxide–carbon nitrate film and the functional groups of cellulose paper, so loss of the catalyst from the supporting electrode is not an issue. The electrocatalyst films are prepared by merging graphene oxide and carbon nitrate nanosheets in dispersion, into which the cellulose fiber paper is dipped.
After drying, the graphene oxide is reduced to graphene using hydrazine vapor, with the porous structure of the cellulose paper allowing efficient penetration of the vapor into the film.The resulting composite film is can then be used directly as a working electrode in an alkaline OER reaction. The porous film allows for a large amount of water to be adsorbed onto the hydrophilic surface, and the excellent charge and mass transfer across the porous network leads to efficient dissociation of the water molecules by the carbon nitrate. Performance of the film is comparable to that of the conventional benchmark catalyst for the OER reaction, InO2, while the hybrid film is highly stable over long-term use, due to its flexibility and strong bonding between the catalyst and support.
This composite electrocatalsyt shows great promise as an alternative to typical electrocatalysts, particularly due to its long term stability and low cost of production, using readily available materials. The simplicity of this novel electrocatalyst should also lead to similar developments for other energy harvesting, conversion, and storage reactions important for a range of renewable energy technologies.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.