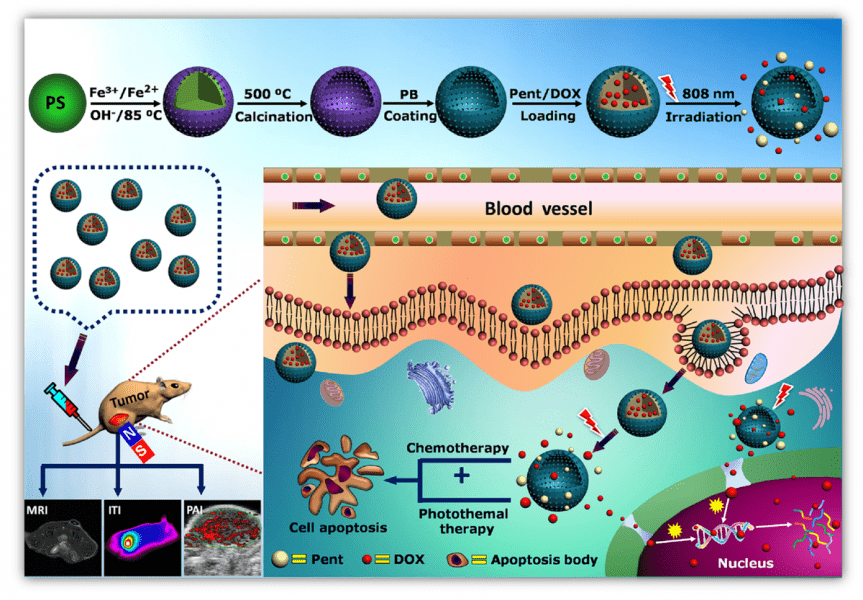
Malignant tumors are considered to be among the most lethal diseases worldwide on account of the aging and boom of propagation. Anti-tumor drugs encapsulated in theranostic nanoparticles for in-site tumor therapy exhibit significant advances because of their relatively high therapeutic outcomes and low adverse effects. However, the potential leakage of anti-tumor drug during the transport in circulation in vivo is a great concern in this field, leading to unavoidable toxic side effects on healthy tissues/organs. Therefore, design and development of a nanocarrier for targeted drug delivery with near “zero release” property, through a safe, simple and efficient approach are very important for tumor therapy.
In a recent article published in Advanced Healthcare Materials, Professor Kaiyong Cai from Chongqing University and colleagues have applied nanotechnology to develop a precisely “zero release” controlled drug delivery system, which demonstrates great potential for the inhibition of tumor growth with tri-modal imaging of magnetic resonance imaging (MRI), photoacoustic tomography imaging (PAI) and infrared thermal imaging (ITI). It was constructed by employing a biocompatible phase-change material of 1-pentadecanol (Pent) as “Drug Janitor” and loading into hollow magnetic Prussian blue nanoparticles, yielding HMNP-PB@Pent@DOX particles. With this design, the release of anti-tumor drug DOX was triggered by near-infrared (NIR) irradiation. Since Pent is at solid phase under physiological condition (37 °C), the “pent-up” DOX in the drug vehicle would not leak until the phase-change material transfers to the liquid phase (>42 °C), which is caused by the increased temperature upon irradiating the photothermal drug carrier with NIR laser. This means the as-designed system could achieve near “zero release” for on-demand drug delivery so as to reduce the toxic side effects of anti-tumor drug during treatment.
The design in this study provides a new strategy for developing near “zero release” drug delivery system and engineering of available theranostic anti-tumor nanoplatform. For more information, check out the paper here.