 Precise control of the primary, secondary, and tertiary structure is a significant new development in synthetic polymer chemistry. Using techniques inspired by other disciplines, it is now possible to synthesize a variety of monodisperse, sequence-defined a variety having diverse applications. Synthesis involving dormant reactive groups, excesses of bifunctional monomers, chemoselective ligation, and multicomponent reactions, as first applied in organic synthetic chemistry, biochemistry and supramolecular chemistry is now being successfully applied in polymer chemistry. Researchers in France have developed a chemoselective method of producing sequence-defined oligo(triazole amides)s and oligomers, confirming the trend towards fine control in molecular design and controlled polymerization.
Precise control of the primary, secondary, and tertiary structure is a significant new development in synthetic polymer chemistry. Using techniques inspired by other disciplines, it is now possible to synthesize a variety of monodisperse, sequence-defined a variety having diverse applications. Synthesis involving dormant reactive groups, excesses of bifunctional monomers, chemoselective ligation, and multicomponent reactions, as first applied in organic synthetic chemistry, biochemistry and supramolecular chemistry is now being successfully applied in polymer chemistry. Researchers in France have developed a chemoselective method of producing sequence-defined oligo(triazole amides)s and oligomers, confirming the trend towards fine control in molecular design and controlled polymerization.
Monodipsersity and precise control of sequence and structure is critical in certain new polymer science applications, e.g., data storage. Using ideas from organic synthetic chemistry, a large library of monodisperse oligothiophenes can be produced using latent reactive groups. To avoid extraneous polymerization, the monomers contain a dormant functional group, which is activated only after the monomer is attached to the main polymer chain, allowing for the addition of further monomers. Thus, long chain polymers can be built from successive linking and functionalization steps.
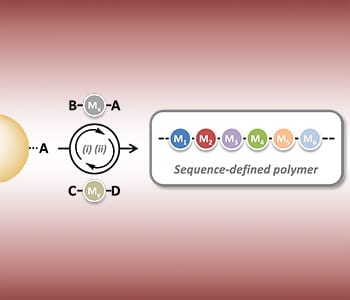
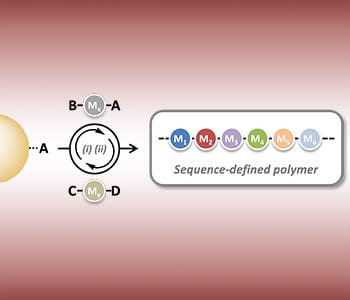
An alternative method—termed the AA + BB strategy—is the successive coupling of two different monomers, one doubly terminated with A groups, and the other doubly terminated with B groups, using the monomers in large excess as compared with the initiator. The excess of the monomer ensures that only one of the two monomer groups is coupled to the polymer chain. Similarly, the AB + CD strategy is another method using two different building blocks. In this case, one monomer is terminated with A and B groups, the other with C and D, and chemoselective coupling steps ensure that group A couples to group C, and group D to group B. Cycling these steps allows the building of long polymers of defined length and structure, through the use of different AB or CD monomers at different stages. Successful synthesis of monodisperse sequence-controlled peptides, oligo(triazole amide)s, and oligomers has been demonstrated using such a strategy. An intermediate approach—combining concepts from the AA + BB and AB + CD strategies—has been applied to dithiol and allyl acrylamide building blocks, AA and BC monomers respectively.
Finally, monodisperse and sequence-defined oligomers have also been synthesized via a novel, multicomponent reaction involving three different sub-building blocks: AB, C, and DE molecules. Successive three-component linking reactions lead to the building of a monodisperse oligomer of desired length. This multicomponent strategy has also been extended to four-component reactions.
The methods described above do not require a protecting group during synthesis, and thus avoid the time-consuming and experimentally demanding coupling, capping, and deprotection steps. However, these methods have their own drawbacks. For the most part, the manual synthesis times for each of the procedure is long, which limits the length of the polymer sequences obtained to around 5–20-mer, and the novel synthesis routes can be experimentally demanding themselves. On the other hand, these synthesis strategies lead to high coupling yields, and monodisperse products, comparable with biopolymers that are able to perform advanced tasks. Optimization of these procedures—with rapid and efficient coupling steps, and automated synthesis—will allow scaling of these techniques, and open up precision polymer chemistry for exciting new developments in applied polymer science.
The Best of Macromolecular Journals Edition 2016 is a special reprint issue highlighting the most impressive contributions from over one thousand articles published across the Macromolecular Journals family in 2015. All of these articles are free to access for one year. Find out more at www.best-of-macros.de.