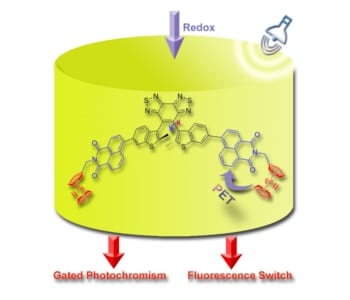
Researchers from China present a ferrocene-grafted photochromic triad that can act as multifunctional switch. Its fluorescence properties as well as its photochromism can be modified with chemical or electrochemical redox reactions.
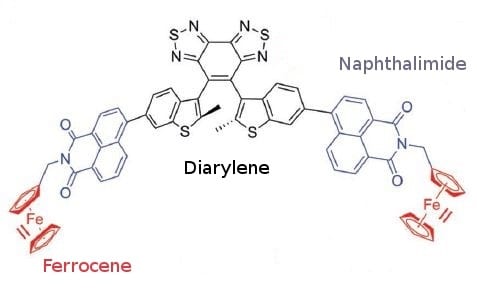
Structure of the photochromic triad with diarylethene bridge, naphthalimide flourophor and ferrocene unit.
Photochromic molecules and materials change their absorption spectra upon irradiation. This property enables their utilization in applications such as molecular switches and sensors. Diarylethenes show photochromism due to switching between their open and closed form via photocyclization and cycloreversion. The closed form is generally colored, while the open form is colorless. They are widely used to study photochromism because of their fatigue resistance, and since the color change cannot be achieved thermally.
In their work, Wei-Hong Zhu and co-workers combine a diarylethene unit with a naphthalimide chromophore and a redox-active ferrocene. Introducing ferrocene leads to gated photochromism of the diarylethene unit. No photochromism can be observed in the presence of the ferrocene complex (Fe(II)) while there is enhanced photochromism after its oxidation to ferrocenium (Fe(III)). As a consequence, a redox-active gated photochromism is achieved.
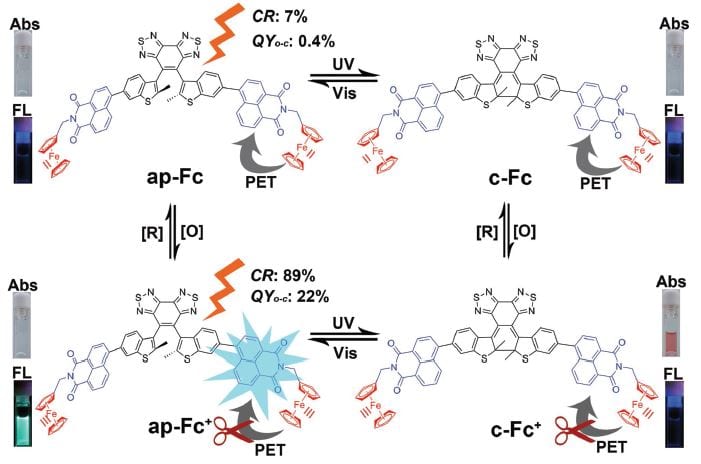
Multiple states and switching mechanism of the photochromic triad.
Further, oxidation and reduction of the ferrocene/ferrocenium unit leads to “ON” and “OFF”-switching of the naphthalimide fluorescence. Ferrocene as an electron donor quenches the naphthalimide fluorescence via photoinduced electron transfer. After oxidation to ferrocenium, the fluorescence properties of the naphthalimide unit are restored.
With their photochromic triad, the authors develop a system that can be manipulated by light, chemical, and electrochemical stimuli. They propose that this multi-addressable triad can be a platform to develop multifunctional molecular switches and multiresponsive materials.