Anthropogenic CO2 emissions caused by the combustion of fossil fuels for electricity generation and transportation are the primary cause of global warming.
Catalytic CO2 hydrogenation to high-value hydrocarbons represents an innovative strategy for reducing both CO2 emissions and fossil fuel consumption, but typically requires harsh temperature and pressure conditions (e.g., 250–450 °C, 2–5 MPa) to achieve meaningful CO2 conversions. Compared with traditional thermal technologies, solar-driven CO2 hydrogenation is a far more attractive strategy since solar energy is free and accessible in most parts of the world.
Tierui Zhang, Technical Institute of Physics and Chemistry (Chinese Academy of Sciences), Geoffrey I.N. Waterhouse, The University of Auckland, Xiaodong Wen, Institute of Coal Chemistry (Chinese Academy of Sciences) and their teams, successfully demonstrated a vibrant new catalyst platform which enables the production of valuable chemicals and fuels from CO2 using a photothermal synergistic strategy.
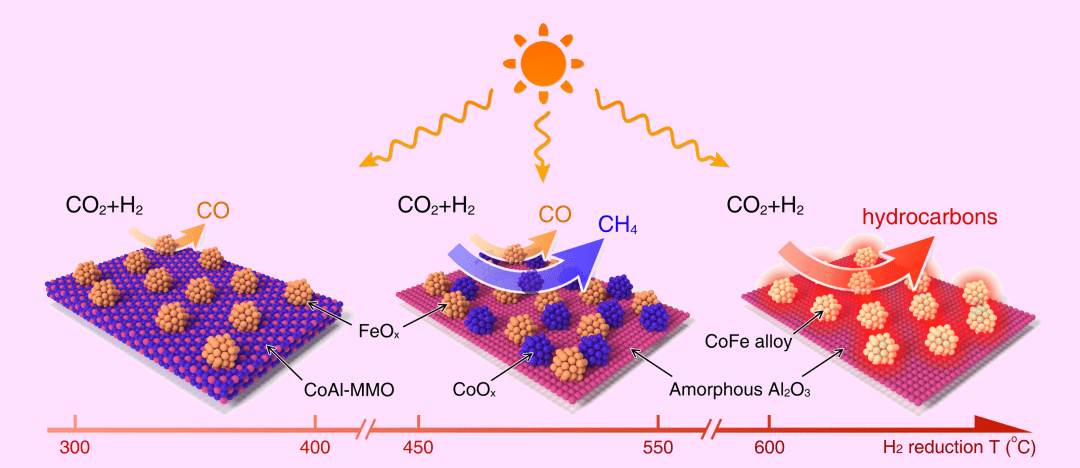
Three unique CoFe-based catalysts were fabricated by direct H2 reduction of a CoFeAl layered-double-hydroxide (CoFeAl–LDH) nanosheet precursor at different reduction temperatures up to 700 °C.
Thechemical composition of the reaction products was found to be highly dependent on the temperature, and the conversion of CO2 increased with UV–vis irradiation time.
LDH precursor reduction at temperatures above 600 °C results in the formation of alumina-supported CoFe–alloy nanoparticles, which demonstrated very high CO2 conversions and remarkable selectivity (≈ 95%) toward hydrocarbons (including >35% selectivity to C2+ hydrocarbons) under simulated solar excitation.
Density functional theory calculations also confirmed the key role of the CoFe alloy nanoparticles in promoting C–C coupling reactions during CO2 hydrogenation.
This work establishes a completely new approach for harnessing abundant solar-energy to produce high-value hydrocarbons from a CO2 feedstock.