Oxygen provided to each and every cell in our body by the lungs is essential for life. We suffocate when its supply is interrupted even for a few minutes, which can occur when we suffer from illnesses that cause respiratory distress.
Acute respiratory distress can occur in many scenarios. For instance, a pre-term baby whose lungs aren’t fully developed does not have enough capacity to supply all the oxygenation needs of its body. Similarly, adults afflicted with respiratory infections, such as the recent COVID-19 caused by the SARS-CoV-2 virus or the previous SARS-CoV virus, also suffer due to the infection incapacitating a portion of the lungs from effective exchange of gases.
In such conditions, mechanical ventilation where oxygen gas is forced into the lungs can only provide support in less severe cases and often has other long-term complications. Therefore, an alternative way of introducing oxygen into the blood is required to effectively treat patients under respiratory distress, to keep their body functioning while relieving the stress on their lungs and allowing them to recover.
Such a technology has been used in cardiac surgeries as part of the heart-lung machine, which supports “out-of-body” cardiac and respiratory support to patients whose heart and lungs are unable to carry out gas exchange to spread oxygen around the body. The technique is called extra corporeal membrane oxygenation (ECMO) and supports the patient for short durations of time. During the last SARS pandemic, ECMO was used to support severely ill patients suffering from acute respiratory distress, allowing recovery of the lungs in over 70% of cases. Nevertheless, ECMO is an expensive, resource intensive technology that is bulky and requires support only available in hospital settings, such as skilled trained personnel for its operation and a reliable oxygen supply.
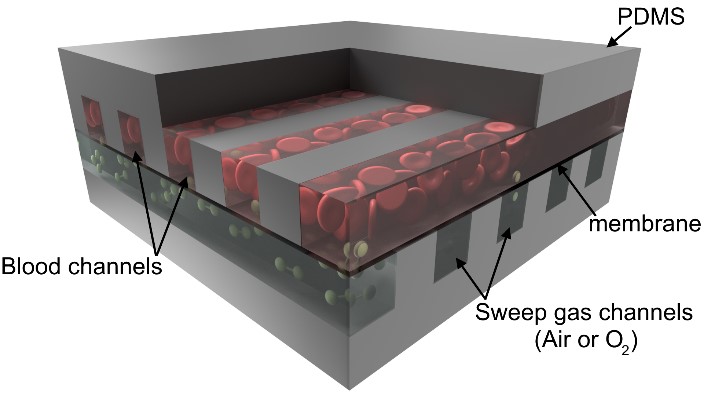
In our lungs, highly branched networks of blood vessels sheathed by a thin membrane provide a large surface area for exchange of gases between the blood and ambient air that we inhale. Oxygenators are compact medical devices that perform a similar task by providing a high surface area interface that can allow such an interaction to occur. In these systems, ambient air is pulled into the machine from its surroundings and purified to provide as much oxygen to the patient as possible, connected via a major artery in adults or through the umbilical artery (often referred to as an artificial placenta) in pre-mature babies.
Over the past decade, advanced microfabrication methods that are used in the electronics industry have been utilized to make microfluidic oxygenators that mimic the highly branched, thin, membrane-like vascular networks found in our lungs. Through microfabrication, extremely thin area membranes can be reliably fabricated, which allows rapid exchange of oxygen and carbon dioxide. Microfabrication methods also enable construction of synthetic vascular networks that mimic systems, much like the branched blood vessels in our body that provide smooth flow paths and reduce chances of blood clot formation. Moreover, researchers have produced oxygenators that are compact, have small filling volume, which is important to avoid the need for blood transfusion and associated complications when connecting them to vulnerable patients, especially pre-term babies.
These microfluidic oxygenators can be perfused solely by the heart, much like our lungs, thereby simplifying their operation.Newer designs developed over the past 5 years have the ability to oxygenate from ambient atmosphere, which makes the device completely passive. These developments promise the realization of a passive and truly portable artificial lung that can overcome many of the issues associated with the current ECMO system, and can be of benefit in resource poor and remote settings.
Future developments in this field of research include extending the findings from the laboratory to clinical practice through extensive testing. Another key area of focus is the development of effective anti-coagulation coatings that allow the artificial lungs to operate over long periods of time without adverse reactions of the blood. New 3D-printing methods are being explored to produce biomimetic hierarchically branched vascular networks with associated membranes similar to the natural lungs in order to reduce the size of these devices even further.
The development of these technologies and their validation is expected to significantly impact the survival and health of a significant section of vulnerable populations, both young and old, affected by respiratory distress. Use of artificial lungs can provide an alternate treatment method to current practice in treating patients affected by respiratory distress, such as some of the COVID-19 patients in severe condition.
Reference: Mohammadhossein Dabaghi, et al. ‘Miniaturization of Artificial Lungs toward Portability.’ Advanced Materials Technologies (2020). DOI: 10.1002/admt.202000136