Advances in nanotechnology affect various parts of our daily life, and nanoparticles are ubiquitously present in nume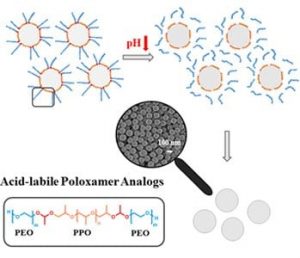 rous applications, ranging from grocery to clothing to pharmaceuticals, a circumstance attributed to the unique properties of particles on the nanoscale. The more specialized the field of application, the higher are standards for purity and definition of a nanoparticle-based system. Miniemulsion polymerization constitutes a potent technique to generate well-defined polymer-based nanoparticles, and further on, facilitates encapsulation of cargo molecules capable of promoting e.g. a specific biological response in terms of a pharmaceutical agent. Elaborate nanoparticle formulations constitute an eminently promising perspective in various fields of research, including cosmetics, coatings and more specialized applications in biomedical fields.
rous applications, ranging from grocery to clothing to pharmaceuticals, a circumstance attributed to the unique properties of particles on the nanoscale. The more specialized the field of application, the higher are standards for purity and definition of a nanoparticle-based system. Miniemulsion polymerization constitutes a potent technique to generate well-defined polymer-based nanoparticles, and further on, facilitates encapsulation of cargo molecules capable of promoting e.g. a specific biological response in terms of a pharmaceutical agent. Elaborate nanoparticle formulations constitute an eminently promising perspective in various fields of research, including cosmetics, coatings and more specialized applications in biomedical fields.
A major challenge for nanoparticle formulations obtained from miniemulsion polymerizations, however, derives from the indispensable use of surface-active agents (surfactants) required to stabilize the emulsions during polymerization. Since high amounts of surfactant are generally essential for an effective stabilization and removal of surfactant from the final nanoparticles is only possible to a limited extent, overall performance of the resulting nanoparticle formulation is strongly governed by the properties of the additive. Moreover, surfactants often convey negative environmental impacts and, in some cases, exhibit toxic behavior. This drawback, to date, limits the utility of nanoparticles generated by miniemulsion polymerization for high performance applications.
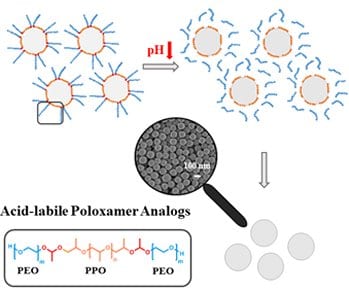
Matthias Worm, Holger Frey and co-workers (Johannes Gutenberg-University Mainz, Germany) now introduce a novel class of nonionic surfactants containing acid-labile junction points and demonstrate a convenient strategy to exploit pH-sensitivity to cleave the surfactant after the polymerization, permitting to efficiently remove surfactant fragments from the nanoparticles. These prototype polymeric amphiphiles are composed of a poly((ethylene oxide)-b-(propylene oxide)-b-(ethylene oxide)) (PEO-b-PPO-b-PEO) triblock substructure in analogy to poloxamers with two acid-sensitive acetal moieties at the block junctions. Acid-triggered precipitation facilitates removal of surfactant fragments from the nanoparticles, which simplifies purification and enables nanoparticle precipitation “on demand. This new class of surfactants has great potential in the development of highly pure nanoparticle formulations and the design of pH-responsive nanocarrier systems.