A diabetic foot ulcer (DFU) is a skin wound that occurs mainly on the bottom of the foot and affects approximately 20% of diabetic patients. Diabetic neuropathy, poor circulation, and a weakened immune system, which worsens when sugar levels are not adequately controlled, put individuals with diabetes at greater risk of developing DFU. These factors make it difficult to detect and heal wounds under the feet, often leading to infections, amputations, and even death in severe cases.
Current treatments of DFU require dressing removal to analyze the wound visually and sometimes sampling to check the presence of pathogens or measure other vital parameters. Besides requiring trained staff and specific tools, this procedure is time-consuming and accurate prognosis is difficult.
Smart sensors integrated into bandages that monitor some physiological parameters of the wound in real time have been developed to overcome these hurdles. However, they use bulky electronics and wires that prevent their use in body areas with high compression and shearing, like the feet.
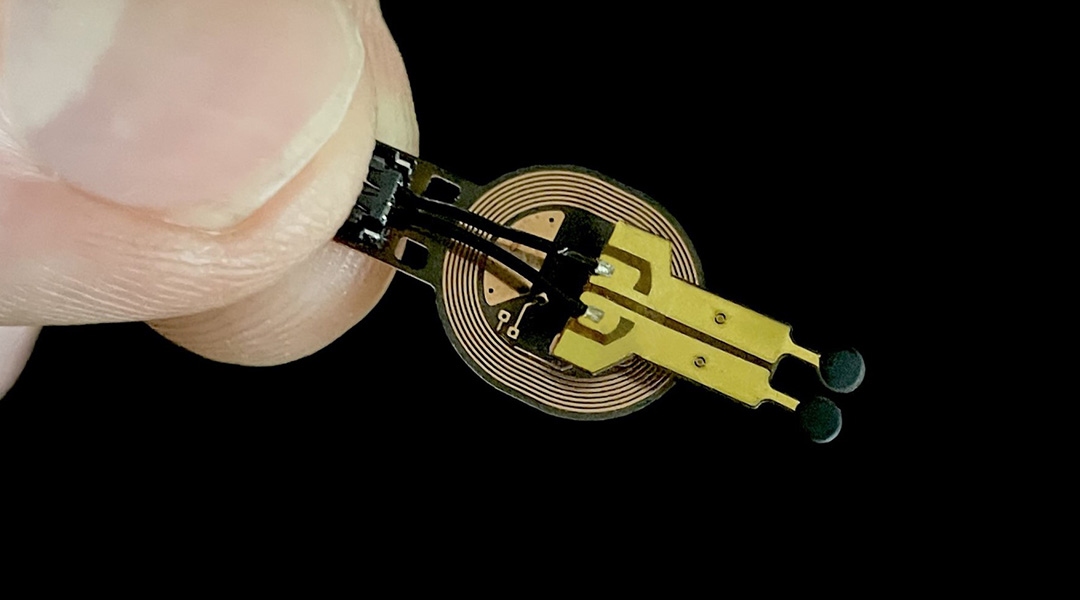
To address this, a group of researchers from North Carolina State University in collaboration with Northwestern University, IBM, Columbia University, and Beth Israel Deaconess Medical Center at Harvard Medical School recently developed a battery-free wireless miniaturized sensor that monitors wound status by measuring the levels of a metabolite called lactate and use them to predict if wounds will have a normal or deficient closure.
The device was able to classify wounds as normal or showing signs of impaired healing in a rodent model of diabetes with high accuracy within the first three days post-injury. This early prediction of healing will enable accurate and timely treatment of wounds, which is crucial in preventing the progression of DFU or other chronic wounds into more severe forms.
Designing the sensor
Lactate is a byproduct of glucose fermentation in mammalian cells. The team decided to monitor this metabolite in the wounds as it controls several wound healing processes, such as inflammation, the formation of new blood vessels, and re-epithelization. Lactate levels within 5 to 15 mM are associated with wound healing, while levels out of this range delay or hinder recovery.
“The sensor is inspired by biofuel cell technology”, explains Amay J. Bandodkar, Assistant Professor at North Carolina State University and one of the researchers behind the sensor´s design. “Biofuel cells produce a voltage signal that reflects the concentration of the target chemical without the need for external power supplies”, which allowed the team to get rid of the batteries that are typically used in sensors.
Lactate is both the analyte and fuel for the cell, oxidizes at the anode, releasing negative charges. These travel through a circuit to the cathode. The flow of charge generates an electrical current converted by a resistor into a voltage signal directly related to lactate concentration.
To make the sensor specific for lactate, the scientists immobilized lactate oxidase in the anode, an enzyme that specifically promotes lactate oxidation. In the cathode, they used silver oxide to receive the charges released by lactate. An electrical circuit of a conductive material connects the anode and cathode.
To further decrease the sensor’s size, they replaced Bluetooth with nearfield communication (NFC) for data transmission to an external reader like a computer or a smartphone. “NFC technology is also used in wireless phone charging and contactless card payment machines. The advantage over Bluetooth is that it requires less energy for data transmission and has simpler electronic circuits”, explains Bandodkar.
The scientists covered the sensor with thin membranes of PVC and chitosan, a bio-polymer derived from crustaceans, to prevent the diffusion of reagents to the wound and protect the sensor. They added edible charcoal to preserve the anode´s enzyme from UV light damage commonly used to sterilize medical devices.
Testing the sensor at the lab
To begin with, the scientists assessed how the sensor reacts to lactate. They found the sensor responds fast and consistently to changes in lactate levels ranging from 0.47 to 30 mM, which allows measurement of lactate levels optimal for efficient wound healing (5-15 mM) and also non-optimal levels. Also, they confirmed that the sensor is specific for lactate as it did not respond to other common wound metabolites such as glucose or uric acid.
The wound bed is a complex environment containing various molecules and fluids that could interfere with detection or damage the sensor. The team found that pH values —a measure of acidity— common in the body do not affect the sensor´s sensitivity. As a final check, they carried out biocompatibility assays, confirming that the device is not toxic for mammalian cells and is secure to use in the body.
Collecting data and training the sensor
After setting up the sensor, the team used it in a classical wired mode to investigate how lactate levels correlated with wound closure rate in healthy and diabetic rodent models.
In both groups, lactate levels peak three days after injury, with most of the lactate values of healthy mice falling within the optimal range for wound healing, and most of the diabetic mice measurements being above.
To set up the predictive use of the sensor, the scientists incorporated a mathematical model into the system that after training on the data, could classify wounds as healthy or deficient healing.
After training, the model showed an accuracy of 76 % in predicting wound healing using the lactate levels in the wound as the only data. Impressively, this accuracy raised to 86 % when they added data on wound pH. This early prediction of wound closure allows early removal of the sensor, avoiding adhesion of the new tissue to the sensor and tissue damage.
Bandodkar clarified that the sensor needs to be validated in larger animal models and clinical trials with humans before reaching commercialization.
Reference: Nate Garland et al., A miniaturized, battery free, wireless wound monitor that predicts wound closure rate early, Advanced Healthcare Materials (2023). DOI:10.1002/adhm.202301280.