 Short interfering RNA (siRNA) has become an important tool in molecular biology and is a promising drug for a variety of diseases. Because siRNA cannot cross cell membranes by itself, siRNA needs a transport vehicle to deliver it to target cells. However, common transfection reagents have drawbacks such as being cytotoxic and lacking specificity. Therefore, new approaches to siRNA delivery are of great importance.
Short interfering RNA (siRNA) has become an important tool in molecular biology and is a promising drug for a variety of diseases. Because siRNA cannot cross cell membranes by itself, siRNA needs a transport vehicle to deliver it to target cells. However, common transfection reagents have drawbacks such as being cytotoxic and lacking specificity. Therefore, new approaches to siRNA delivery are of great importance.
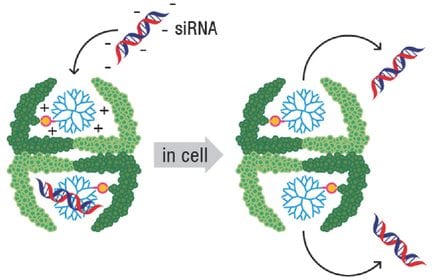
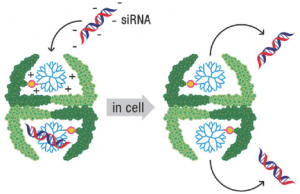
In a new Open Access article in Advanced Science, Nico Bruns and co-workers from the University of Basel and University of Fribourg, linked a polymeric transfection reagent into a hollow protein nanocage, where it was still able to bind siRNA. This approach enabled to protect siRNA from early degradation, lowered the toxicity of the transfection reagent and specifically deliver siRNA into a certain type of cancer cells. Additionally, they showed that by modifying the surface of the protein with a cell-penetrating moiety, the siRNA could also be transported into another cell line.
Thus, protein cages that contain nucleotide-binding polymers within their cavity represent powerful and versatile delivery vehicles that combine the properties of polymeric transfection reagents with the uniform size and unsurpassed functionalization possibilities of protein nanocapsules. In the future the protein cage-polymer conjugate could be additionally modified with targeting moieties to transport siRNA, or other drugs, to specific cells.