Enzymatic biofuel cells hold great promise in generating electricity without the need for noble metals. The enzymes used in these cells can be mass produced and can even process organic fuels like methanol and sugars. One of the main challenges for researchers is how to transfer electrons efficiently from the active site of the enzyme, which may be deep within the protein, and the electrode. Chemicals that transfer the electron to the electrode can be used (mediated electron transfer) but direct electron transfer (DET) is preferable, especially if it is important that the fuel cell remains nontoxic.
Covalently linking enzymes to multi-walled carbon nanotubes results in high DET in biological cathodes, but to harness electrical energy from a redox reaction, the electrons must be transported from the enzymes redox center along the tubular network of the MWNTs to the current collector. By using carbon nanosheets rather than nanotubes to support the enzyme, a US research team show in Advanced Energy Materials that electrons can be transferred easily thanks to the open planar structure of the nanosheets.
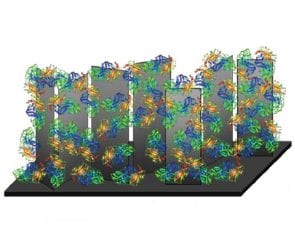
In their new paper, Ramaraja Ramasamy, Zhiguo Zhou, and co-workers developed a high-preformance cathode for the oxygen reduction reaction. Cabon nanosheets (vertically aligned 2D graphitic sheets) were the catalyst support and buckypaper (made up of single-walled nanotube bundles) was the substrate electrode. Carbon nanosheets were deposited onto the buckypaper and formed a complex multi-dimensional structure, a 3D porous coating. This unique structure allowed a multi-copper oxidase enzyme, laccase, to be immobilized onto the surface through the π- π interactions between the tethering agent and the planar walls of the nanosheets.
This strong interaction keeps the enzyme close to the support and reduces the distance that the electron must tunnel from the redox center to the nanosheet. The many graphitic edge-plane sites of the vertically aligned nanosheets encourage electron tunneling. Because the nanosheets are so closely bound to the buckypaper, once an electron tunnels from the enzyme to the closest nanosheet, it is equivalent to reaching the current collector.
The performance of the electrode based on carbon nanosheets surpassed that of electrodes based on multi-walled nanotubes. This development may have dramatic implications for biofuel cells and bio-electrocatalysts.