Differentiating and tracking various cell populations is of high interest to understand their interactions in complex biological systems. Following such systems in vitro and in vivo over weeks could help address key questions in cancer research, cell differentiation, cell therapy, regenerative medicine, and embryogenesis.
Recently, the implementation of multiple functions within a nanoparticle (NP) has been used. However, the implementation of multiple functions within the same NP entails complex synthetic procedures, which cannot always achieve high reproducibility of the NPs properties. The cost,effectiveness and toxicity of these NPs remains to be the addressed.
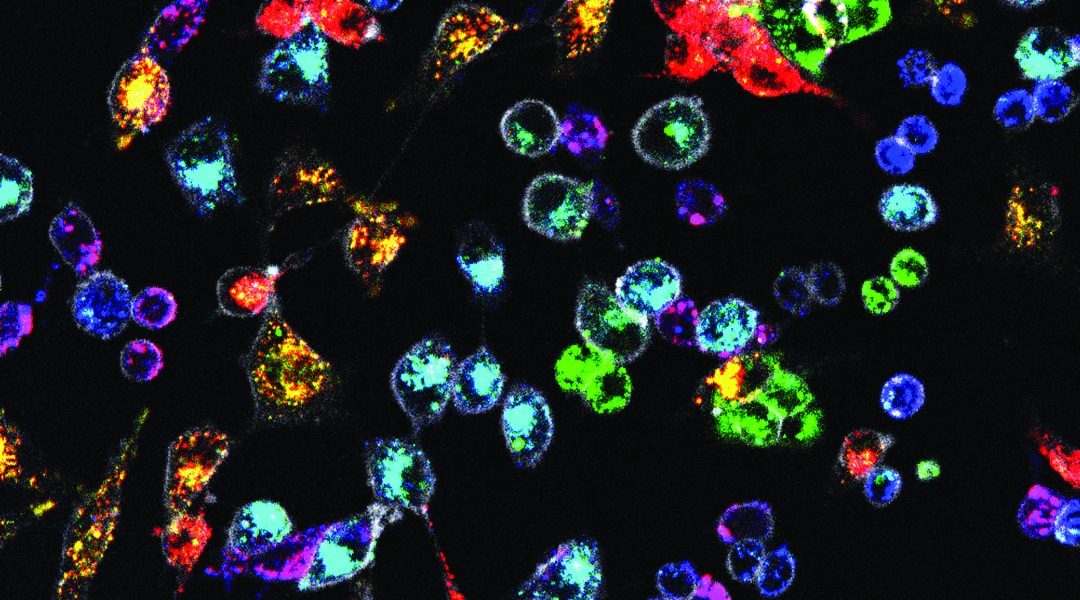
In recently published research, Andreas Reisch, Andrey S. Klymchenko, and co-workers from France, developed a method that allows long-term labeling of different cell populations in tens of different colors using fluorescent nanoparticles. Their approach is based on a set of 3 dye-loaded nanoparticles made of the biodegradable polymer PLGA. The nanoparticles contain one of three cyanine dyes, DiO, DiI or DiD, with distinct excitation and emission colors, which are efficiently encapsulated at high concentration using bulky fluorinated counterions. The resulting 40 nm particles are 20-fold brighter than quantum dots of similar color. The three types of nanoparticles have thus different colors but identical sizes and surface properties, which makes their interactions with biological systems identical.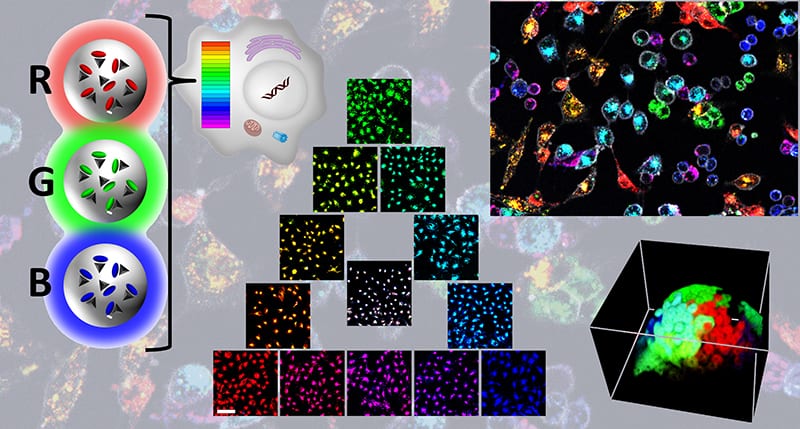
The three types of nanoparticles are equally well endocytosed by numerous cell lines, leading to a homogeneous staining of a whole cell population. Mixing nanoparticles of the three colors in different proportions can then be used to create a homogeneous RGB barcode in cells, which is faithfully transmitted through many cell generations.
Different cell types labelled in this way could be easily distinguished in cocultures of various cell lines over weeks. In zebrafish, cancer cells coded in different colors could be easily tracked in vivo, while direct microinjection of these particles in zebrafish embryos was used to monitor morphogenesis in six different colors.
The developed approach provides a valuable tool for studying complex systems in vitro and in vivo without their genetic modification.
Kindly contributed by the Authors, and edited by Jodie Haigh.