 This article originally appeared on the Ceramics Tech Today blog of the American Ceramics Society.
This article originally appeared on the Ceramics Tech Today blog of the American Ceramics Society.
GPS is a great tool. It can guide you turn-by-turn to your city’s shopping mall. It is, in fact, an “in situ” device and observes your location in real time. Once there, however, you will need to go inside and look at the mall’s directory to find the Apple store (c’mon, we know where you were going). The GPS won’t get you the whole way there, but it will get you into the region where you can find what you are looking for.
A paper that was published recently in the online Early View of the Journal of the American Ceramic Society by Bolon and Gentleman demonstrates a GPS-like use of polarized confocal Raman spectroscopy to study ferroelastic domain transformation in ceria-stabilized zirconia. They were interested in whether or not a Raman technique could be used for in situ, nondestructive characterization of thermal barrier materials, a common application of zirconia-base materials.
Lots of research has been reported on toughening of zirconias. Transformation toughening—increased toughness resulting from the tetragonal to the monoclinic phase change—is the most common toughening mechanism. However, transformation toughening runs up against the realities of the phase diagram and the crystallographic transformation does not occur above about 800°C.
Toughening that occurs above 800°C is thought to be the result of ferroelastic domain formation and switching. Bolon and Gentleman define a ferroelastic material as one that contains “at least two energetically equivalent orientation states and it must be possible to move between those two states through the application of an external load.”
In the case of polycrystalline tetragonal zirconia, the c-axis orientation is equally distributed between the x, y and z directions. When a load is applied, the grains where the c-axis is aligned parallel to the load undergo a reorientation so that the c-axis becomes perpendicular to the load. This is the ferroelastic switching mechanism. To increase the toughness of tetragonal zirconias, energy is absorbed through the reorientation of the tetragonal domains in the presence of a stress field (from the applied load). The stress required to trigger domain switching is the coercive stress.
Raman spectroscopy is usually used as a bulk characterization tool. In this study, the researchers used polarized confocal Raman spectroscopy to observe ferroelastic domain formation in the surface of the material. The Raman images, in effect, map out the regions where ferroelastic switching occurred under load. Those regions can then be looked at more closely using other techniques like TEM.
The authors studied polycrystalline 18-mol% ceria-stabilized zirconia. Two methods for applying loads were used: Vickers indentation and uniaxial compression in a diamond anvil.
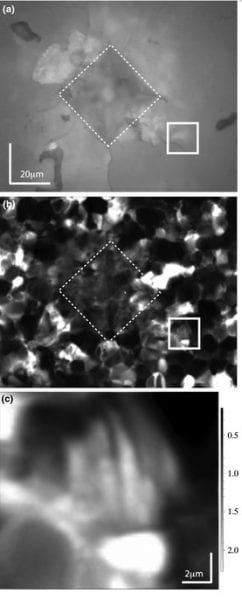
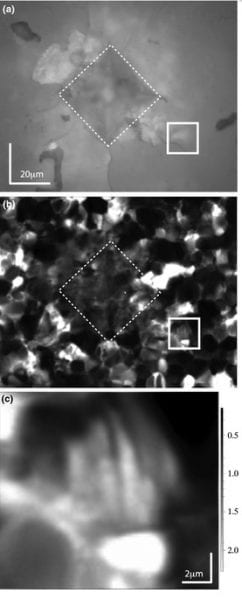
In the illustration above, the top is a white-light image showing the Vickers indent with a dashed line (click on image to enlarge). Cracks can be seen emanating from the indent, and the solid box shows a crack slashing across the lower left quadrant. The middle image is the Raman image. The bright spots are grains where the c-axis is perpendicular to the image (i.e., the computer screen) and the dark regions are have c-axes oriented in the plane of the computer screen. The bright regions along the crack in the solid box are indicative of ferroelastic switching. In the center of the solid box, some faint banding can be seen, which is also characteristic of ferroelastic switching. The lower image shows the banding feature in more detail.
The paper goes into a fair amount of detail regarding Raman intensities and the details of mapping them and how the Raman technique was successfully applied to both load scenarios. The authors conclude that, indeed, the “results provide the first step toward the in situ observation of ferroelastic domain formation” for tetragonal zirconia-based materials. Like a GPS, it can guide researchers in real time to the part of the material where the action is, but different tools are needed for up-close characterization.