 Nature is a rich source of inspiration for researchers designing innovative new materials. Photosynthetic bacteria meet their energy needs using complexes of dyes, called bacteriochlorophylls, embedded in protein and lipid matrices. Using a limited number of dyes, bacteria efficiently harvest the spectral energy available in their habitat by manipulating dye self-assembly. The dyes form ordered aggregates with varying spectral properties to collect and funnel light energy of different wavelengths.
Nature is a rich source of inspiration for researchers designing innovative new materials. Photosynthetic bacteria meet their energy needs using complexes of dyes, called bacteriochlorophylls, embedded in protein and lipid matrices. Using a limited number of dyes, bacteria efficiently harvest the spectral energy available in their habitat by manipulating dye self-assembly. The dyes form ordered aggregates with varying spectral properties to collect and funnel light energy of different wavelengths.
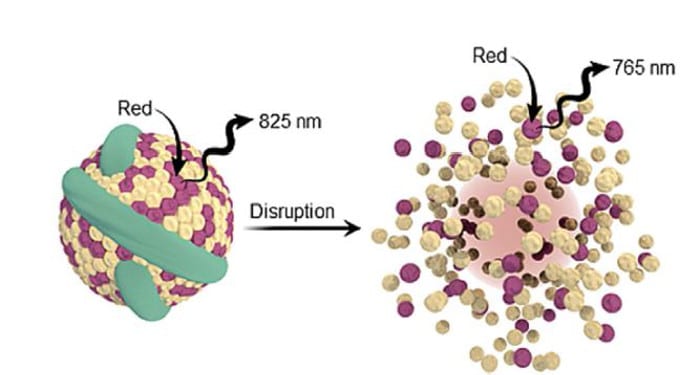
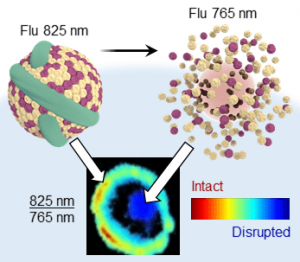
Researchers at the University of Toronto and the Princess Margaret Cancer Centre have imitated this biomaterial by incorporating bacteriochlorophyll within a lipoprotein nanocarrier (published in ChemNanoMat). The aggregated dye fluoresces at 825 nm, while emission is shifted to 765 nm when the nanocarrier is disrupted. This nanostructure-dependent dual-wavelength emission is a new photophysical mechanism for evaluating nanostructures. Using ratiometric fluorescence imaging bacteriochlorophyll aggregation distinguishes intact and disrupted nanocarriers within biological environments.
Systemically administered nanomedicines encounter many biological barriers before they reach their target and exert the desired effect. Nanomedicine behavior including stability, cell uptake, and subsequent drug release as it encounters these barriers can be monitored by bacteriochlorophyll aggregation. Self-assembly is a flexible, dynamic, and responsive strategy for manipulating fluorescence that is applicable to other nanocarrier architectures. Using a single dye also reduces complexities for clinical translation compared with current photophysical mechanisms requiring two distinct dye populations. Bacteriochlorophyll aggregation is a powerful new tool for drug delivery and theranostics due to its simplicity, robustness, and versatility.
This article is part of a special issue on Nanobiointerfaces in ChemNanoMat. Click here to see the other articles in the issue.