The spinal cord plays an essential role in sensory processing and controlling movement, breathing, and other voluntary and involuntary body functions. As a component of the central nervous system, it communicates with the brain and other parts of the body through its numerous nerve bundles. Therefore, spinal cord injuries have serious consequences, including paralysis.
Given its complexity and poor regeneration capability, successful reconstruction of the spinal cord is a difficult undertaking. Biological scaffolds for this purpose must meet specific requirements: good biocompatibility to promote the adhesion of nerve cells; a high water content to meet the needs of cell metabolism; a highly permeable, oriented 3D fiber structure to facilitate cell migration and guide axonal extension; and good flexibility to resist deformation under various stresses when the spinal canal is opened.
Hydrogels, the most frequently used tissue engineering scaffold, address some of these requirements, but in order to mimic the axon fibers of the spinal cord, the processing technique must also be taken into account. Electrospinning offers a means to achieve directional fiber structure at the micro- and nanolevel.
Using electrospinning technology, a group of researchers have constructed a new hydrogel scaffold for spinal cord regeneration. The scaffold is based on gelatin methacryloyl (GelMA), which is both biocompatible and photo-crosslinkable, imparting mechanical tunability and stability.
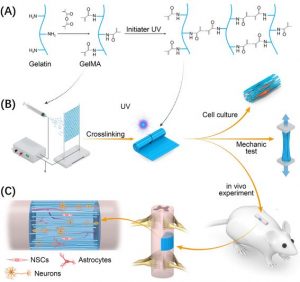
Fabrication of the GelMA scaffold and evaluation in a rat model.
The researchers assessed the in vivo performance of the scaffold on rats impaired with hindlimb dysfunction as a result of spinal cord injury. At 12 weeks, the GelMA group demonstrated a significantly better recovery compared to the control group and gelatin scaffold group—in fact, there was no significant further improvement in hindlimb function after 12 weeks, which suggests that the regeneration process was essentially completed over this period.
Immunohistological evaluation confirmed the long-term survival of neural stem cells, as they were still present in the GelMA scaffold after 12 weeks. Astrocyte formation, on the other hand, dominated in the gelatin scaffold group. Astrocytes lead to the formation of glial scars, which hinder the extension of axons.
Newly formed blood vessels at the injured site can also trigger axon regeneration. A large number of vascular regenerations were also found in the GelMA scaffold group compared to the control and gelatin groups, further confirming its suitability in treating spinal cord injuries and perhaps other types of injured soft tissue.