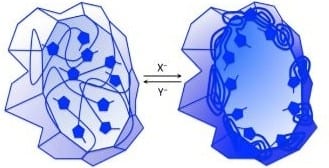
New polymeric materials are being designed that change their physical or chemical properties when exposed to heat, light, or, in particular, chemical components. These changes include opening and closing of pores in thin films and membranes, condensation and dissolution of colloidally stable nanoparticles, swelling and shrinking of materials in one, two and three dimensions, and the controlled dispersion of functional fillers in composite material matrices.
An important component of this expanding field is being provided by polymers derived from ionic liquid monomers that are liquid organic salts at temperatures below 100°C. The largest class of such monomers comprises cationic imidazolium groups ion paired with various anions. This moderate-temperature liquidity imparts interesting chemical properties on the resultant polymers and materials that derive from how these imidazolium-anion pairs interact with themselves and with various solvents.
Series of anions have been found to tune some of these changeable properties over concentration levels having a dynamic range of over 104. Further insight into how such functional polymers and materials behave will accompany further developments in our understanding of how imidazolium-anion pair solvation can be influenced by various intensive fields such as temperature, chemical potential, and electromagnetic fields.