When the first immunotherapy cancer treatments became available in the late 20th century, the idea that the body’s own immune system could be harnessed to destroy tumors brought hope to millions of patients.
For some, immunotherapy can be truly life-saving, but for others, the treatment that at first offers hope can become a devastating disappointment — immunotherapy doesn’t work for everyone and some cancers simply don’t respond to it.
Researchers have spent decades trying to make immunotherapy a viable treatment option for every cancer patient, and researchers at the Salk Institute for Biological Studies may have discovered one piece of the puzzle: Mitochondria, the organelle best known for producing the chemical energy that powers our cells.
“There are two things cancer cells need to do,” said co-senior author Gerald Shadel, professor in the Molecular and Cell Biology Laboratory at the Salk Institute. “They need to grow uncontrollably, and they need to evade the immune system.”
A changing understanding of mitochondria
Shadel, who collaborated with immunologist Susan Kaech on a study recently published in Science, said scientists used to think mitochondria didn’t play a part in the way cancer cells grow. Today, they know that mitochondria actually affect tumor growth in multiple ways.
Shadel, Kaech, and their colleagues wondered if it was also possible that the mitochondrial function in cancer cells might change in a way that helps the cells evade the immune system. To find out, they manipulated the way electrons travel through the mitochondria in mouse melanoma cells.
Mitochondria use electrons to make a molecule called adenosine triphosphate (ATP), the cell’s energy source. Electrons can travel through the mitochondria in two different ways. “We were surprised to find that when you force the electrons away from one complex and into another, that actually led to enhanced immune recognition by the T-cells that killed the tumor,” said Shadel.
The results could have significant implications for cancers with a particular immunoevasion strategy. “A very common way that tumors evade the immune system and are refractory to immunotherapy is they actually downregulate [major histocompatability complex] (MHC) expression,” said Shadel. “MHC is a cell surface molecule that presents the antigens to the T-cells.”
When the body’s T-cells — a type of white blood cell — encounter these antigens, they recognize the cells as foreign and destroy them.
When the researchers changed the way the electrons traveled through the mitochondria, it caused a build-up of a metabolite called succinate. The succinate moved into the cell’s nucleus, where Shadel said it “upregulated the machinery in the tumor to present antigens on the surface”.
Kristine Willis, program director at the National Cancer Institute’s Division of Cancer Biology who was not involved in the study, said the research has “a lot of potential to give us some insights into how we might make immunotherapy more generalizable”.
But she also cautioned that because melanoma cells tend to be naturally more responsive to immunotherapy, similar research needs to be done on different tumor models.
Still hurdles to overcome
A lot of things have to happen before the discovery will have therapeutic applications. Scientists will also need to figure out how to manipulate electron transport in cancer cells without affecting energy production in healthy cells. Then, they will have to develop a drug that can achieve the same results in humans.
“The actual therapeutic modality would be either a small molecule that rewired the mitochondria in the way we’re talking about or targeting something like [the methylation-controlled J-protein, which is a mitochondrial protein that interacts with the transport of electrons],” said Shadel. “I think the third way is actually even more easy to understand. Maybe we can just give succinate.”
Willis added that the paper is a reminder that cancer is not just about mutations. “It’s also important to remember that cancer is also a disease of cells and cell metabolism,” she said.
Mitochondria are best known as the cell’s powerhouse, but Shadel said they’re more complicated than most people realize.
“They do many more things than just make ATP,” he said. “There’s a myriad of possibilities of how to modulate mitochondria in different ways for different potentially therapeutic endpoints. Not just cancer, but other diseases too.”
Reference: Gerald Shadel, et al., Manipulating Mitochondrial Electron Flow Enhances Tumor Immunogenicity, Science (2023). DOI: 10.1126/science.abq1053
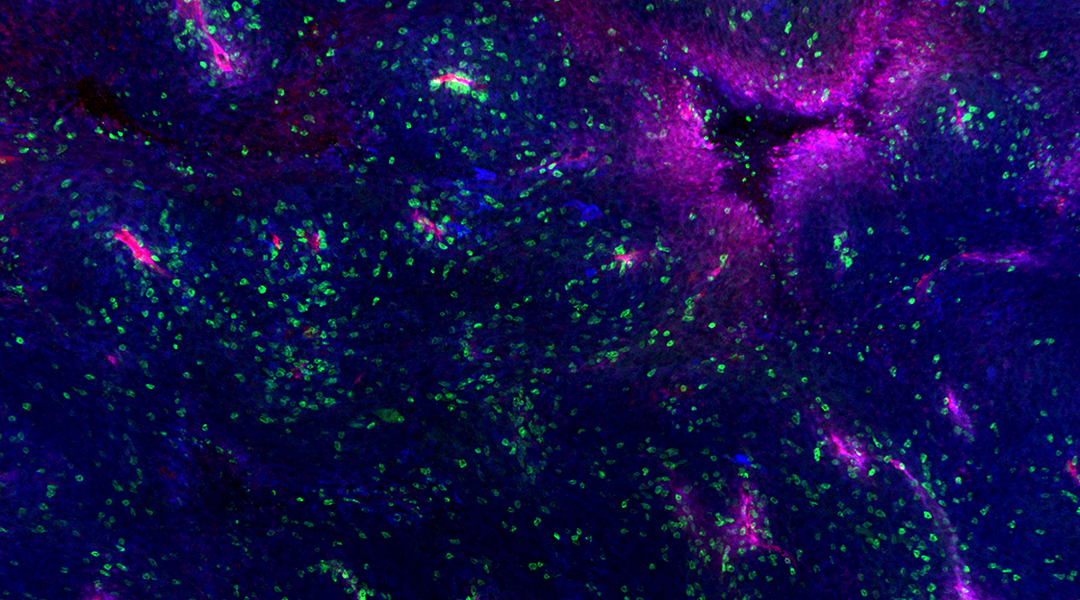
Feature image: A mouse melanoma tumor showing blood vessels (red), MHC proteins (blue), and an infiltration of specialized immune cells (green). Credit: The Salk Institute