From a myriad of data obtained from experiments in mouse models, human stem cells, clinical blood samples, and human cell cultures, new research published in the journal Advanced Science suggest that nitric oxide changes the functioning and structure of neurons and leads to autism-like behaviors.
Autism spectrum disorders (ASDs) comprise a vast group of neurodevelopmental conditions united by symptoms such as poor social skills, communication difficulties, repetitive behaviors, and niche hobbies. Several mutations are responsible for the diversity of features associated with ASD, but the mechanisms underlying them are little understood, leaving patients with no available therapies.
Nitric oxide has also been shown to play a versatile role in various neurological disorders, such as Alzhiemer’s, Parkinson’s, and Hungtinton’s diseases, but its role in ASD has remained obscured. Recently, Haitham Amal, a principal investigator and assistant professor at The Hebrew University of Jerusalem, in Israel, and colleagues reported finding that mouse models of ASD had significantly high levels of nitric oxide.
“Important to note is that we still don’t know if [nitric oxide] leads to ASD as a first source,” said Amal. “We now know that [nitric oxide] is a major pathological factor in ASD. We also know that reducing [nitric oxide] levels reduce ASD phenotypes.”
The role of nitric oxide
As part of a new study, Amal’s team conducted a series of experiments using cellular and mouse models of ASD as well as biological samples from patients to further characterize the potential role of nitric oxide in the development of autism.
Within the brain, normal levels of nitric oxide are responsible for blood flow, neuronal growth and metabolism, among other functions. But at increased levels, the molecule can be toxic, disrupting cellular processes.
When researchers injected typical mice with a nitric oxide donor — a substance that releases nitric oxide — the mice displayed molecular and behavioral changes that were indicative of ASD. “Our findings show that [nitric oxide] affects the expression of key neuronal proteins that are important for brain development. We believe that [nitric oxide] leads to the degradation of these proteins,” said Amal. In response to high levels of nitric oxide, some neuronal proteins were produced in excess, while the making of others was quashed.
Mouse models of ASD are known to have a lower density of dendritic spines on their neurons. These spines are small bumps present on branch-like protrusions, called dendrites that help receive inputs from other neurons. The researchers found similar changes in the structures of neurons exposed to more nitric oxide — their dendritic spines were scarcer. Moreover, the mice exhibited a variety of behaviors linked to ASD, including little interest in novel objects, weaker social memory, and more anxiety.
Given that abnormal levels of nitric oxide led to atypical changes in the levels of neuronal proteins and the structure of neurons, the researchers wondered if the molecular and behavioral features of autism could be reverted by inhibiting nitric oxide in mice.
Can the effects be reversed?
To test this theory, the team used two different mouse models of ASD — one with a mutation in the SHANK3 gene and another with a mutation in the CNTNAP2 gene, both of which have been found to be important in autism, with well-established mouse models available. As expected, both mouse models showed signs of autism. For instance, the density of dendritic spines dropped significantly compared with normal mice.
The researchers injected the mice with an inhibitory chemical that would suppress the production of nitric oxide. As levels of nitric oxide fell, the signs of autism tapered off. Normal levels of neuronal proteins were restored, and dendritic spines returned at typical density. “In an ASD state, the number of spines is reduced, and after treatment we found an almost full recovery,” explained Amal.
Furthermore, the nitric oxide inhibitor also negated the autism-like behaviors otherwise seen in these mouse models. “The ASD mouse models with high [nitric oxide] levels showed social deficits, reduced novelty seeking, repetitive behavior and anxiety,” added Amal. “When we reduced the NO levels, we found reversal of most of the features.”
After having tested their hypothesis in living mice, the researchers turned their focus to cell cultures. To begin with, they cultured neuronal cells from normal and mutant mouse models. Increasing and decreasing levels of nitric oxide in these cultures led to similar biochemical changes as those seen in experiments with mice.
Having investigated the impact of nitric oxide in mice, Amal’s team sought to confirm their findings in humans. First, they tested neurons that were derived from the stem cells of people with mutations in the SHANK3 gene, living with ASD. These neurons had high levels of proteins that help diagnose stress caused by nitric oxide. When researchers treated these neurons with a nitric oxide inhibitor, the levels of these proteins subsided.
Thereafter, Amal’s lab measured the levels of the same proteins in samples of blood plasma taken from children with ASD. They wanted to validate their results in this demographic. Compared with unaffected children, those with ASD had higher levels of biomarkers that indicate nitric oxide stress.
Deeper analyses revealed that the production of numerous proteins responsible for neuronal development was increased or decreased, differing from their normal levels. Further, using computational analyses, the researchers found that genes involved in several mechanisms connected to ASD development were overrepresented. These genes are key to severing connections between neurons as well as driving inflammation and oxidative stress.
“This research is a significant breakthrough in autism research with the first direct connection made between an increase in the concentration of [nitric oxide] in the brain and autistic behavior,” said Amal. “I am hopeful that with our new understanding of the [nitric oxide] mechanism, we can begin to develop therapeutic drugs for ASD and help millions of children and adults living with autism around the world.”
Amal’s team is exploring the impact of nitric oxide in many more models of autism. “The good news is that we are exploring very similar data,” added Amal.
Reference: Haitham Amal, et al., The NO Answer for Autism Spectrum Disorder, Advanced Science (2023). DOI: 10.1002/advs.202205783
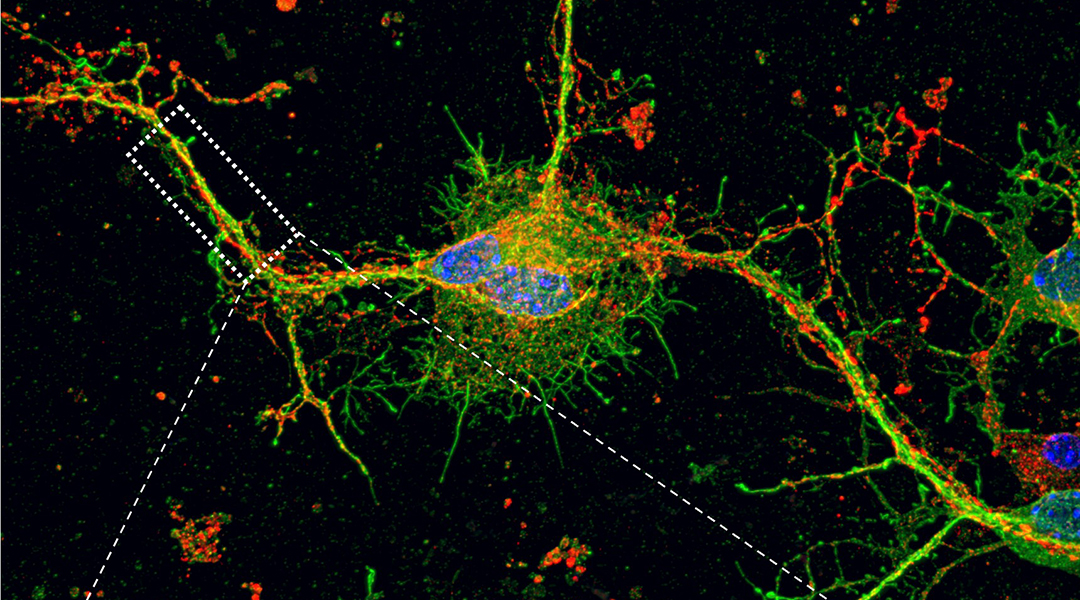
Feature image: A confocal microscopy image of synaptophysin expression in Shank3 mutant primary cortical neurons treated with nitric oxide inhibitor, which provides valuable insights into the potential role of nitric oxide in modulating synaptic protein expression.