 Nanomedicine is an ever-growing field with many developing and predicted therapeutic applications, such as for multiple sclerosis, atherosclerosis and cancer. SPIONs, golden nanocages, carbon nanotubes and graphene nanoribbons are only few examples of what is now available to combat often devastating and potentially fatal diseases. In addition to biomedical applications, nanoparticles (NPs) are widely used as electronic components, in manufacturing of automotive and industrial paints, plastics and food color additives, therefore increasing our exposure to them on a daily basis. As the field grows, so do the concerns of their toxicity.
Nanomedicine is an ever-growing field with many developing and predicted therapeutic applications, such as for multiple sclerosis, atherosclerosis and cancer. SPIONs, golden nanocages, carbon nanotubes and graphene nanoribbons are only few examples of what is now available to combat often devastating and potentially fatal diseases. In addition to biomedical applications, nanoparticles (NPs) are widely used as electronic components, in manufacturing of automotive and industrial paints, plastics and food color additives, therefore increasing our exposure to them on a daily basis. As the field grows, so do the concerns of their toxicity.
However, most studies address the synthesis and R&D processes for new nanomaterials, and only few of them focus on the potential health hazards of NPs. Published nanotoxicology research studies implement various in vivo, in vitro, in silico and pharmacokinetic assays to study the interactions of NPs with cellular components and their effect on important cellular functions (mitochondrial activity, integrity of cell membranes, apoptosis and necrosis, etc.). Unfortunately, the nanoscale size of NPs equips them with some biophysical properties that make them incompatible with traditional assays, reducing their sensitivity and interfering with their results. Short-term and long-term effects of NPs on our bodies, therefore, remain unknown, creating an urgent need in modified or novel nanotoxicity assays that are able to overcome these technical limitations and provide us with much needed answers as to whether new nanotechnologies are safe for us and our environment or not.
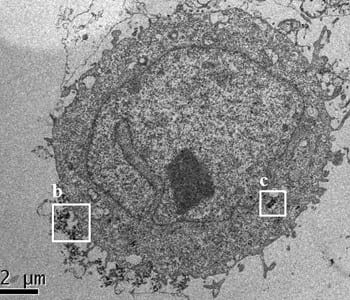
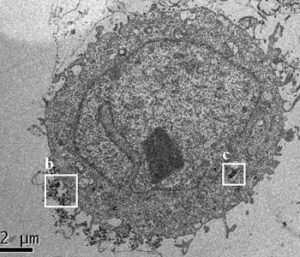
Image cytometry and flow cytometry (FCM) are designed to analyze cells on a single-cell level. Cytometry techniques can detect natural side scattering intensities of individual NPs, making them simple, sensitive and cost-effective candidates for assaying the biological effects of NPs and evaluating their toxicity. These methods were successfully used to determine the biological activity of Fe3O4, TiO2, and Ag NPs. In new work, Tae Hyun Yoon and co-workers combined FCM with the X-ray fluorescence (XRF) technique to study the interactions of SiO2 NPs with HeLa cells. The cells were exposed to SiO2 NPs with different core diameters, hydrodynamic sizes, and surface charges. The researchers then proposed an empirical equation based on the observed relationships among these experimental data for the determination of cellular SiO2 NPs from their normalized side scattering intensity (nSSC) as well as core diameter.
The combination of these methods offers a simple and relatively inexpensive semiquantitative tool to estimate cellular NP contents and provide insights into cell-NP interactions, opening new possibilities for a clearer understanding of their potential toxicity and side effects.