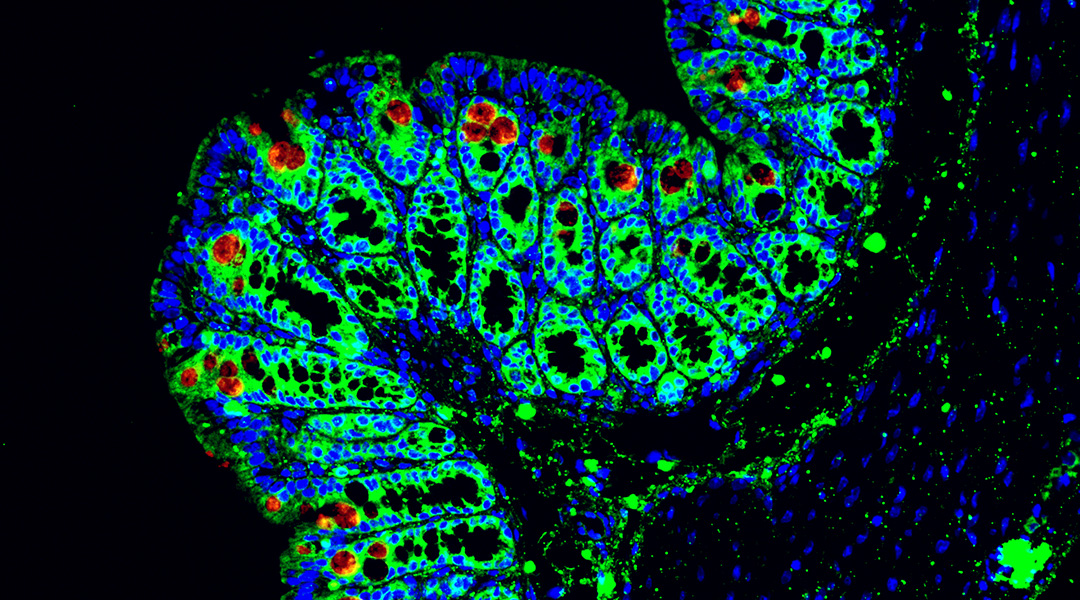
An imbalance in our gut’s natural bacteria could be a major underlying cause of inflammatory bowel disease (IBD) in people with a rare metabolic disorder known as glycogen storage disease (GSD).
GSD is a genetic disorder with a frequency of roughly 1 in 20,000 to 43,000 births. It affects how the body stores and uses sugar for energy, namely in the liver and muscles. Under normal conditions, the body converts glucose into a molecule called glycogen — a stored form of glucose that acts as a backup energy source which can be quickly converted to keep blood sugar levels stable and provide energy.
Due to genetic alterations in people with GSD, enzymes responsible for the break down of glycogen back into glucose are dysfunctional, leading to a build-up of glycogen in the liver and muscles. This can cause a host of health issues, including low blood sugar levels, muscle cramps, fatigue, and an enlarged liver.
A curious subtype of glycogen storage disease
But what intrigued a research team led by Min Yang, professor of pediatrics at the Southern Medical University in China, was the fact that a set of GSD patients with a subtype of the disease known as GSD-lb also suffer from chronic IBD. “We have been concerned about GSD-associated IBD for nearly 10 years,” said Yang in an email.
Little is known about how and why IBD develops in GSD-lb, with greater than 70% of cases presenting severe digestive symptoms that are only occasionally observed in other subtypes of the disease. Previous studies have only made tentative links between IBD, a dysfunctional immune system, and its effects on gut microbiota, but Yang’s team wanted to dive deeper.
“Various innate immune cells maintain [balance in the gut],” they wrote in a paper published in Advanced Science. “However, a deeper understanding of the interactions between gut microorganisms and immune cells is lacking, and the key [molecules] contributing to GSD remains unclear.”
Without a concrete understanding of these mechanisms and the relationship between the immune system and the gut’s microbiome, scientists will be unable to develop effective treatments or interventions for IBD symptoms related to GSD. Yang and her team hope to change this.
Uncovering the missing link
A growing body of research has established the critical role that microorganisms in the gut play in our overall health.
To create a causative link between IBD and GSD, Yang and her team began by assessing how the microbiome had become altered in GSD patients. Their study included 477 fecal samples collected from 150 patients with GSD, 137 family controls, and 190 unrelated healthy controls to help control for multi-variable conditions.
Perhaps unsurprisingly, they found distinctive microbiota that differed compared to healthy individuals. “The prevalence of certain bacteria and detectable colonization of bacteria in the healthy gut were disrupted in patients with GSD,” stated the authors in the article. “31 genera were significantly altered in patients with GSD compared to controls.”
The most significant changes were found in the samples coming from the GSD-Ib subgroup., such as increased levels of harmful bacteria and the presence of oral microbes related to systemic infection—an unexpected finding, according to Yang, since these microorganisms are normally found in the nose, throat, and lungs.
How they got into the GI tract and what role they play in GSD remain unknown at this point.
“The pathogenic bacteria from the oral can colonize and multiply in the intestine, indicating that the intestine provides the conditions and environment conducive to the growth and reproduction of these bacteria groups,” explained Yang. “[In future studies] we expect to find the root cause of oral pathogens colonizing and proliferating in the gut,” said Yang.
Altogether, these findings suggest that interactions among bacteria in GSD create a self-sustaining system. These bacteria likely rely on each other for resources and survival, forming a complex network of mutual support that leads to gut imbalance and dysfunction of the intestinal lining. The team says this dysfunction is potentially caused by the collective harmful activities of the bacteria, but more study is required.
Crosstalk with immune cells
Another important element of the study focused on the “crosstalk” between the gut microbiota and immune cells.
The scientists found that immune cells called macrophages were present in higher levels in biopsy samples taken from GSD-Ib patients compared to control samples. These macrophages release short proteins called chemokines that act as signals to help guide other immune cells to where they are needed.
A deeper analysis identified the significance of a subtype of macrophage that produces the chemokine CCL4L2, which is responsible for activating other immune cells against the growth of harmful bacteria.
While the natural functions of CCL4L2 are typically protective, Yang and the team suspected that excessive or dysregulated stimulation by the chemokine as a result of the bacteria found in GSD-lb patients’ guts could be a possible cause of the IBD symptoms they experience.
This was initially backed up in lab experiments, where macrophages treated with a cell medium obtained from cells infected with the harmful bacteria were more “activated” compared to those treated with a control medium. This activation was then blocked when a CCL4L2 inhibitor was added to the culture, confirming that CCL4L2 might indeed be boosting macrophage activity, leading to an inflammatory response against the bacteria that ends in IBD.
The team then searched for CCL4L2 in biopsy samples taken from GSD-lb patients and found CCL4L2 proteins coupled with a receptor called VSIR, found in gut cell walls. Similar results were found in a mouse model of IBD in which the scientists found higher levels of macrophages compared to healthy controls, and after treatment with an antibody designed to inhibit VSIR, they found symptoms in mice became aggravated.
“Macrophages interact with intestinal epithelial cells through CCL4L2-VSIR ligand-receptor signaling to promote damage repair,” said Yang.
Yang said her team is studying how the CCL4L2-VSIR pathway works and will also explore its potential for developing patient treatments. The team expects that targeting this pathway could provide a therapy beneficial not only for GSD patients but also for those suffering from colitis and other forms of IBD.
Reference: Jiaoli Lan, et. al., Gut Dysbiosis Drives Inflammatory Bowel Disease Through the CCL4L2-VSIR Axis in Glycogen Storage Disease, Advance Science (2024). DOI: 10.1002/advs.202309471