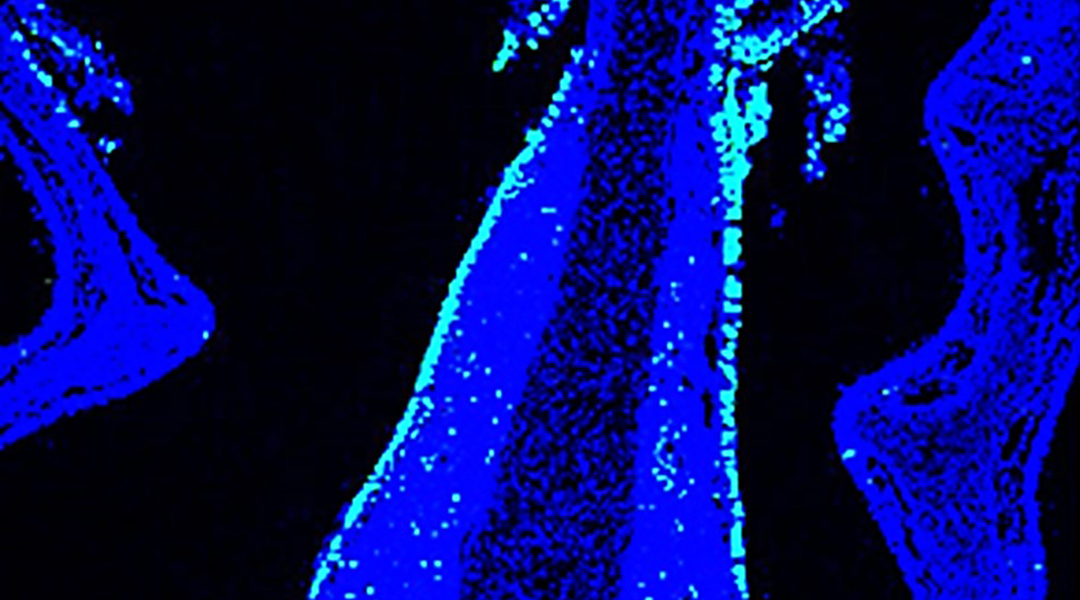
Nasal mucosa (blue) of mouse inoculated with nanoparticulate intranasal vaccine, antigen (green)
Conventional injected vaccines protect from severe disease and can reduce the risk of infection and transmission. We see this in well-established vaccines and in the ones developed recently against SARS-CoV-2.
Intranasal vaccines, though, are thought to have a better chance of breaking the chain of transmission between individuals in viral respiratory infections. The theory here is that if immune cells could stop the replication of respiratory viruses right at the site where they gain entry to establish infection, we could prevent the virus being passed on to the next person.
While vaccines delivered as drops or a spray into the nose hold great promise for protection, their development is fraught with challenges. In a recent paper published in Advanced Science, Korean scientists report a new vaccine design to overcome these.
The same mechanisms that protect us from inhaled particles and pathogens create a barrier to vaccine antigens. Lining the respiratory tract is a layer of mucus that immobilizes foreign material and fine hairs — or cilia — that sweep them back out. In the case of vaccine development, antigens are usually cleaned out of the nasal passage before a robust immune response can be generated.
The research team, led by Professor Kun Na of The Catholic University of Korea, improved the retention of their vaccine in the nasal cavity, and super-charged the ensuing immune response with light. They combined the properties that would make a more effective intranasal vaccine into a nanoparticle held together by electrostatic interactions.
Their nanoparticle complex consists of a harmless viral protein — influenza hemagglutinin — and a light sensitive polymer. Particulate antigens resist clearance from the nasal passage better than smaller proteins. And the positively charged polymer attaches more avidly to the negatively charged surface of epithelial cells that line the mucosa.
Once administered into the nasal cavity, a laser is used to activate the polymer to generate reactive oxygen species. This in turn stimulates the maturation and activation of immune cells and augments the specific immune response against the virus.
“Light activation of nanoparticulate vaccine can act as an adjuvant that allows nanoparticles to better penetrate the intranasal mucosal layer and increase the immune response,” said lead author and graduate student, Hayoon Jeong.
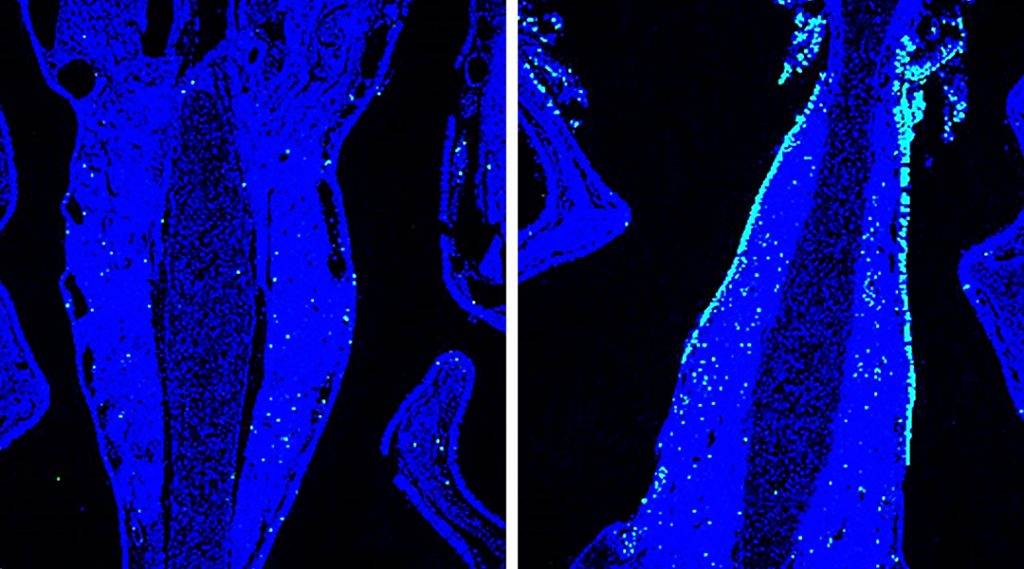
The researchers reported enhanced production of antigen-specific antibodies and effective protection of mice infected with the influenza virus when laser treatment was applied together with the nasal delivery of the nanoparticulate formula.
A needle-free vaccine that generates specific and robust immunity to respiratory viruses could improve uptake of vaccines, especially among people with needle anxiety. Vaccine administration that does not require highly trained staff would also help speed up the roll out of vaccines during pandemics and outbreaks.
“Nasal vaccination has a clear advantage for inducing both local protection in the nasal passage as well as systemic immunity, which will be beneficial for non-respiratory diseases. This vaccine system can be used as a flexible and extendable engineered platform, which can be designed based on specific antigens selected from potential pathogenic infections for efficient vaccination,” said Jeong.
Reference: Hayoon Jeong, et al., Hemagglutinin nanoparticulate vaccine with controlled photochemical immunomodulation for pathogenic influenza-specific immunity, Advanced Science (2021). DOI: 10.1002/advs.202100118