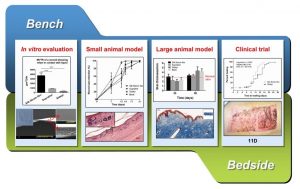
Medical wound dressing is a commonly-used treatment for skin defects. Silk fibroin is a natural protein derived from Bombyx mori cocoons, and shows potential in tissue repair applications due to its excellent biomedical properties. Numerous silk fibroin wound dressings have been developed in the lab, however, lack of large animal studies and clinical trials have hampered their wide use in the clinic.
In a recent study published in Advanced Healthcare Materials, Ouyang, Zou and co-workers from China developed a wound dressing made of silk fibroin film and investigated its application potential for skin repair. The silk fibroin film described in their study is a highly transparent, biocompatible, air-permeable, waterproof scaffold that also acts as a barrier to bacterial infection. An in vivo full-thickness skin defect study showed that the silk fibroin film effectively reduces the average wound healing time with better skin regeneration compared with the commercial wound dressings. Subsequent assessment in porcine model confirmed its long-term safety and effectiveness for full-thickness skin defects. Finally, a randomized single-blind parallel controlled clinical trial with 71 patients demonstrated that the silk fibroin film significantly reduces the time for wound healing and incidence of adverse events in comparison to commercial dressing.
This study presents the first large animal study and randomized controlled clinical trial of silk fibroin film for the treatment of skin defects. It not only paves the way for the clinical translation of silk fibroin biomaterials for skin wound healing, but also gives an example on the translation procedure of biomaterials for researchers who are interested in and wish to take further steps in their own research work.