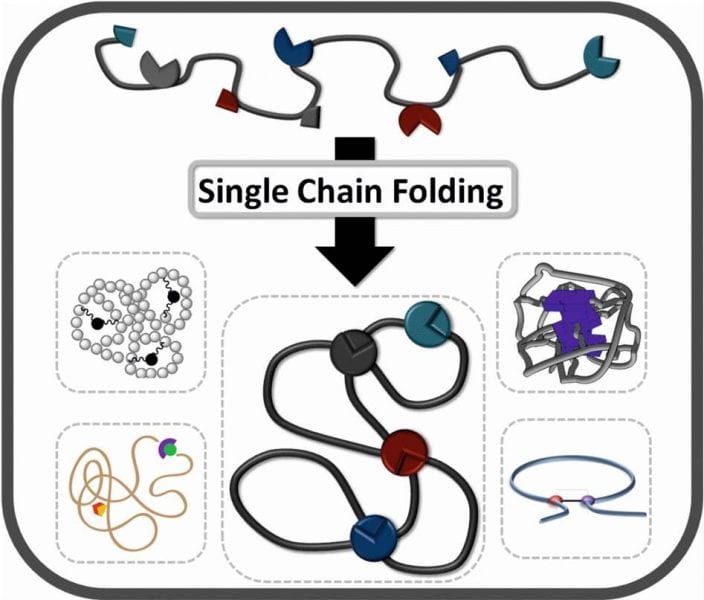
Mimicking the behavior of naturally occurring macromolecules – such as proteins – is one of the key aims of advanced polymer chemistry. Christopher Barner-Kowollik and co-workers (Karlsruhe Institute of Technology) highlight most recent progress in the area of single polymer chain folding, describing approaches where polymer chains are prepared via precision synthesis and are subsequently folded into defined single chain geometries.
Mimicking the behavior of naturally occurring macromolecules – such as proteins – is one of the key aims of advanced polymer chemistry. Christopher Barner-Kowollik and co-workers (Karlsruhe Institute of Technology) highlight most recent progress in the area of single polymer chain folding, describing approaches where polymer chains are prepared via precision synthesis and are subsequently folded into defined single chain geometries.
The obtained geometry is either permanently locked into place via covalent bonds or can be dynamically changed via hydrogen bonding motifs depending on its surrounding environment. The presented analysis of the current state-of-the-art demonstrates that the last 5 years have seen substantial progress in this emerging, vibrant and young field.
Ultimately, the authors see a distinct possibility that true biomimetic synthetic single chain folded polymers carrying biological cues can fulfill a testable biological function – now fulfilled by a natural protein or enzyme – in a bioassay or even a living organism within a timeframe of 10 years. To reach such an ambitious goal a concerted effort is now required between macromolecular chemists, biophysicists and biologists to progress towards true macromolecular biomimetics.
This article is part of the special Best of Macros 2012 issue, and is now free to read at http://www.best-of-macros.de!