From a medical perspective, there are plenty of reasons to be interested in sweat. The quantitative measurement of its components can track certain bodily functions under different conditions and help in the diagnosis of diseases. Chloride concentrations, in particular, are considered an important indicator for cystic fibrosis, characterised by abnormal cellular chloride channels and higher general sweat levels.
As sweat is around 99% water, any other components appear only at very low concentrations, requiring analytical tools to be highly sensitive. The consistent and accurate sampling of a rapidly evaporating liquid appearing in only small amounts from a constantly mobile surface also presents quite an obvious major challenge.
Sweat collection and its secure storage, as well as the analysis of sweat rate and of chemical biomarker components, have now been addressed all at once by a new system designed by a team collaborating across the USA and Japan. The system is able to capture and store sweat released from eccrine glands in sufficient volumes for continuous monitoring and analysis, up to three days later, while avoiding cross-contamination and evaporation issues.
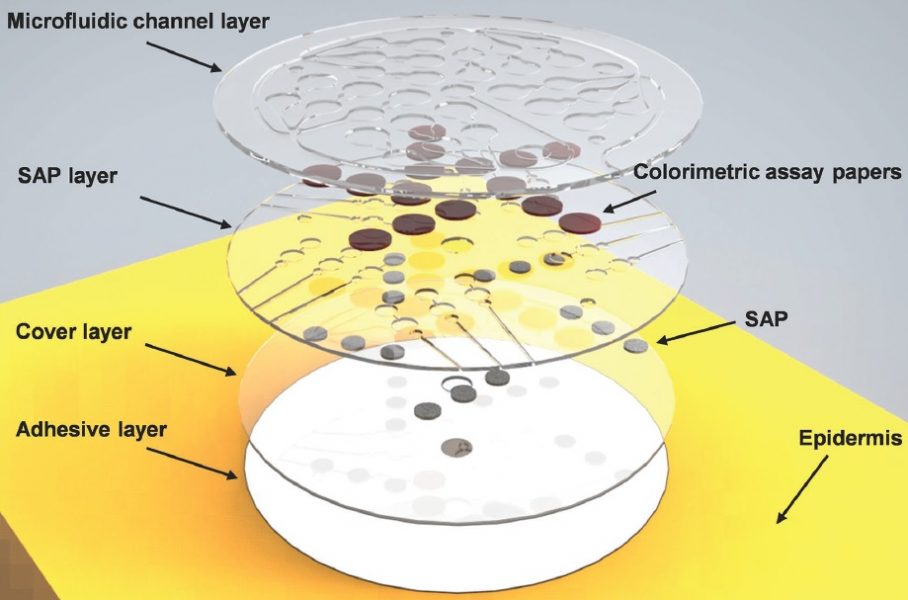
The soft microfluidic device captures and stores sweat, with a sequential valve system allowing the in situ, chronological analysis of samples. SAP = superabsorbent polymer.
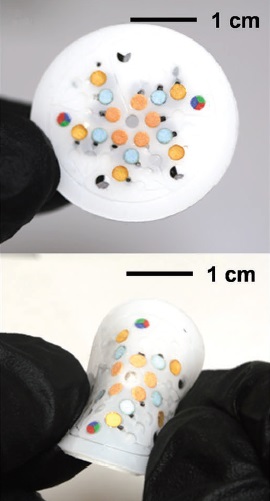
Colorimetric analysis on this flexible device allows a quick visual determination of sweat biomarker content.
The basis of the device is a thin, flexible, microfluidic patch with water-actuated, absorbent polymer valves. The importance of this active valve system, as opposed to the passive valves usually proposed, is that it is not triggered by bending or flexing deformations as the subject moves. This allows the patch to be applied to the skin almost anywhere on the body without compromising its integrity.
The sweat reservoirs fill in sequence to allow chronological tracking, and the in situ analysis of chloride content is made possible via paper discs of colorimetric reagents, tailored to be sensitive and accurate across a broad physiological concentration range.
The colorimetric assays enable a quick visual determination of changing chloride content in the captured sweat when compared to calibration images. “Information should be provided simply, directly, and immediately to the user, while minimizing the necessary steps to infer data with electronic readers or mobile phones,” say the researchers of this study.
Check out the sophisticated sequential valve system and the results of the in vivo tests in their Full Paper in Small.
Interested in microfluidics or health-monitoring devices? Try these articles: