Cancer researchers now believe that targeting the critical energy infrastructure of cancer cells — the mitochondria — may provide a powerful new treatment strategy that overcomes two of the main ways tumors evade destruction.
To kill tumors, doctors use drugs to kill the rogue cells. However, due to the rapid growth of cancer cells and their ability to repair damaged DNA, tumors can quickly develop resistance to these drugs, sometimes spreading throughout the body through a process called metastasis.
Researchers in China believe that by targeting the mitochondria of cancer cells, both these scenarios could be circumvented.
Why mitochondria are a key weakness in cancer cells
Mitochondria are specialized structures inside cells that produce the chemical energy required to keep the cell alive and functioning. According to Yanjuan Gu at The Hong Kong Polytechnic University, recent studies looking into mitochondria have revealed its potential as a cancer therapy target.
“Studies revealed that mitochondria lack robust DNA repair mechanisms, making them vulnerable to targeted therapies,” said Gu.
Mitochondria are contained within a specialized double membrane within the cell. In these compartments are the machinery that carries out the energy production as well as a specialized unit of DNA known as mitochondrial DNA.
One effective treatment against cancer cells is to administer drugs which damage DNA, leading to cell death. But tumors often develop resistance to this because of their DNA repair capability. How veer, the lack of these repair tools in mitochondria prevents this.
Researchers at several institutions found that mitochondria are essential for metastasis — the ability of tumors to spread to other parts of the body. The many mechanisms are still not entirely understood, but changes in tumor cell mitochondria are thought to activate metastasis pathways, where these cancer cell mitochondria can even be transferred to healthy immune cells. This transfer simultaneously suppresses the immune response to the cancer’s spread while also facilitating the spread itself.
Based on these findings, Gu and her colleagues hypothesized that by targeting cancer cell mitochondria, they can avoid resistance and prevent or slow metastasis. The challenge, however, is packaging and delivering drugs to the mitochondria.
Any drug targeting tumor mitochondria must enter the cancer cell and then accumulate in the mitochondria — and nowhere else. To do this, drugs are packaged into carriers called exosomes and specific targeting proteins called ligands are attached. Exosomes are membrane-bound structures used by cells to transport various molecules. Ligands line the surface of the exosome and recognize and bind to the membranes of the cancer cells allowing them to get inside.
Unfortunately, finding the right combination of exosome, ligand, and medicine can be difficult because each component can chemically alter the functioning of the other once they are combined into a single structure.
Developing a smart nanoparticle: OXA@Exo-RD
To address these challenges Gu and her team developed a nanoparticle platform consisting of several components, each with a specific job.
Known as OXA@Exo-RD, it is made up of an exosome, a cancer cell-targeting peptide, a mitochondrion-targeting molecule called dequalinium, and the chemotherapy drug oxaliplatin. The chosen exosome is known to be stable when injected into the bloodstream and is not toxic to healthy tissue.
“[The targeting peptide] is one of the most well-known active targeting ligands evaluated preclinically and clinically and holds promise for targeting cancer cells,” explained Gu. The next two target the mitochondria specifically.
Importantly, dequalinium is positively charged, which serves two functions. In cancer cells the difference in charge on either side of the mitochondrial membrane — known as the transmembrane potential — is much higher than normal cells meaning positively charged particles cross easily and accumulate inside.
Dequalinium, therefore, accumulates in the mitochondria, bringing the anticancer drug with it. It is itself also toxic to the mitochondria, and leads to mitochondria failure and cell death. Finally, the drug oxaliplatin binds to the mitochondrial DNA, causing defects and damage.
Together these components add up to a potent attack on the mitochondria of cancer cells.
To test the nanoparticle treatment, mice with colorectal cancer were treated with OXA@Exo-RD. The team then measured how well the drug entered tumor mitochondria, as well as the reductions in tumor volume and spread from the colon and rectum to the liver.
The results showed that the drug efficiently accumulated in the tumor mitochondria and worked well to reduce tumor size. Compared to control groups, the tumors in mice treated with OXA@Exo-RD were significantly smaller, and when removed and examined, showed the largest amounts of dead cells and necrotic tumor tissue.
Experiments looking at reduced spread of the cancer to the liver found that, compared to control groups receiving saline solution or oxaliplatin on it’s own, treatment with the full OXA@Exo-RD nanoparticle reduce the rate of new tumors in the liver up to 86%.
A step toward more effective cancer treatments
For Gu and the team, these results show that their combination of components making up the nanoparticle are correct. The package was efficiently delivered to the cancer cell mitochondria and worked as expected. But before this treatment becomes commonplace several hurdles remain.
The team needs to evaluate potential side effects to healthy mitochondria and better understand how different tumors will react to the treatment. “Tumor cells exhibit varying mitochondrial membrane potentials,” said Gu, meaning the drug may be less efficient in some cancers. There are also issues with cost, scalability, and regulation.
According to Gu, there are few standardized frameworks for evaluating exosome-based nanodrugs, particularly in terms of immunogenicity and long-term toxicity.
“Regarding cost considerations, the engineered OXA@Exo-RD, as an exosome-based therapy, likely incurs higher upfront costs than conventional chemotherapy due to the complexity of exosome production,” she said. This complexity also reduces the scalability of producing, storing, and transporting the nanoparticles to cancer clinics.
Despite these challenges, “we believe that OXA@Exo-RD represents a promising yet resource-intensive approach for treating chemoresistant cancers,” said Gu. “While current methods, such as conventional chemotherapy, are more accessible, advancements in exosome engineering and manufacturing could help bridge the gap in cost and scalability.”
Reference: Xiaohui Wang et al. A mitochondria-targeted biomimetic nanomedicine capable of reversing drug resistance in colorectal cancer through mitochondrial dysfunction, Advanced Science (2025). DOI: 10.1002/advs.202410630
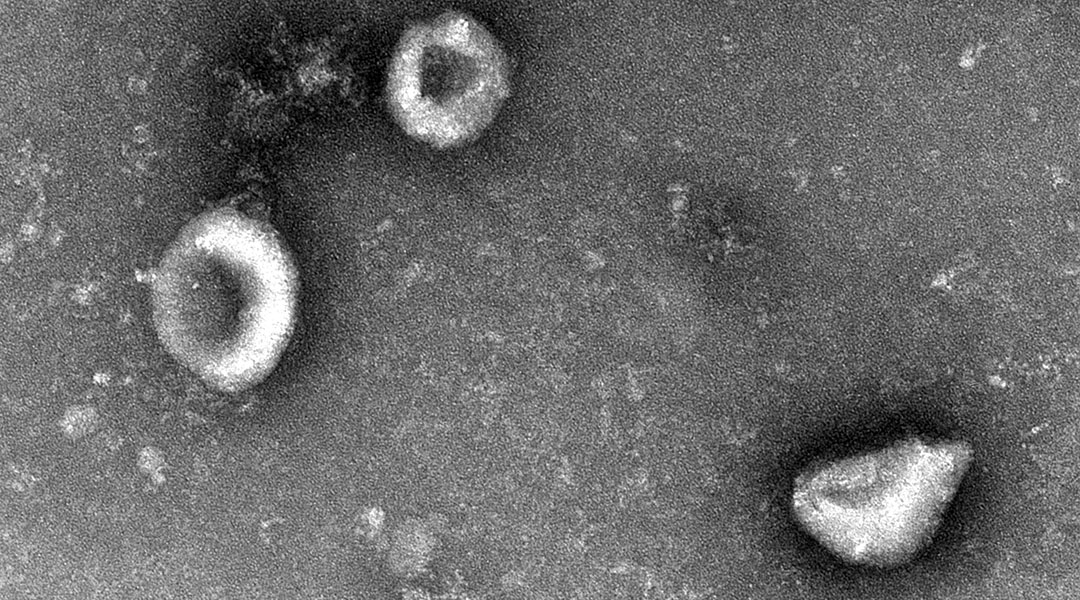
Feature image: Transmission electron microscopy image of the nanoparticles.