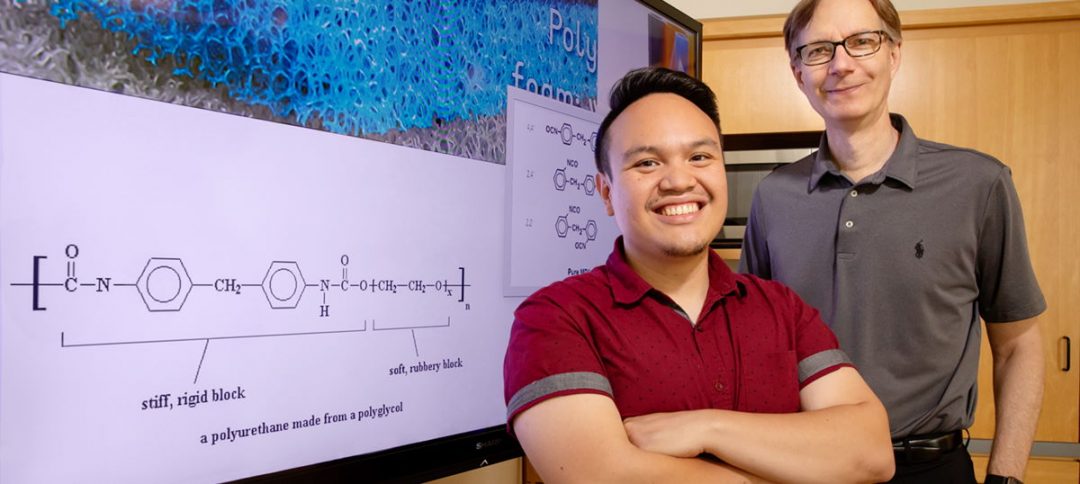
Polyurethane is a polymer formed by reaction of isocyanate with a polyol. These polymers cover an extremely wide range of stiffness, hardness, and density, and are commonly used to manufacture high-resilience foam seating, rigid foam insulation panels, foam seats and gaskets, elastomeric wheels and tires, high-performance adhesives and surface coatings, along with sealants.
In terms of quantity, polyurethane foams are most important as flexible or rigid foams, and the furniture market is its most important sector. The annual global demand for furniture cushions, carpet cushion, and mattresses etc., is more than five million tons.
In addition, as a result of increased use of lightweight and insulation materials in other segments like construction, electronics, and materials for the automotive and packaging industries, there has been a continuous rise in global demand for polyurethane materials in recent years.
Critical polyurethane waste
Polyurethane waste is problematic as numerous hazardous chemicals, such as isocyanates, hydrocyanic acid, and dioxins, are released when these materials are incinerated. Polyurethane products deposited into landfills also decompose into hazardous, environment-damaging substances. To add to this, recycling polyurethane is currently very difficult and energy intensive, as most polyurethanes are thermosetting polymers that do not melt when heated.
With an ever-growing responsibility to turn to more sustainable practices, research has been spent on strategies to enable polyurethane recycling, for example, by mechanical recycling of the polymers (i.e., turning old mattress foams into insulating materials), or through chemical recycling.
With this in mind, a team at the University of Illinois led by Professor Steven Zimmerman has developed a method to break down polyurethane waste and turn it into other useful products.
Change the structure of polyol
Graduate student Ephraim Morado wanted to solve the polyurethane waste problem by chemically re-purposing the polymer. Unfortunately, polyurethanes are extremely stable, which lends to their widespread commercial use, and are made of two components that are hard to break down: isocyanates and polyols.
Polyols are problematic as they are petroleum-based and not readily degradable. To circumvent this difficulty, the team incorporated a more easily degraded and water soluble chemical unit, the acetal.
The degradation products that are formed after dissolving the polymers in a combination of trichloroacetic acid and dichloromethane at room temperature could be repurposed to new materials. As a proof of concept, Morado was able to convert elastomers, widely used in packaging and car parts, into an adhesive glue.
The researchers will report their findings at the American Chemical Society National Meeting and Exposition.
The greatest drawbacks of this new recycling method is the cost and toxicity of the starting materials used to carry out the reaction, Professor Zimmerman has commented. Thus, the researchers are currently trying to find a better and less costly means of achieving the same process using milder solvents, such as vinegar, to carry out the degradation.
While there is still a ways to go, this is an important first step in dealing with a growing environmental problem.