Recent advances in flexible and stretchable electronics establish the foundations for advanced physiological recording systems that mount on the curvilinear surfaces of nearly any region of the body, and on nearly any organ system. Although capabilities in conformal and robust contact are well established through prior studies, materials and designs that minimize or eliminate disruptions to natural diffusive or convective flow of fluids through the device are less explored.
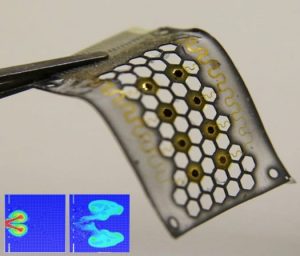
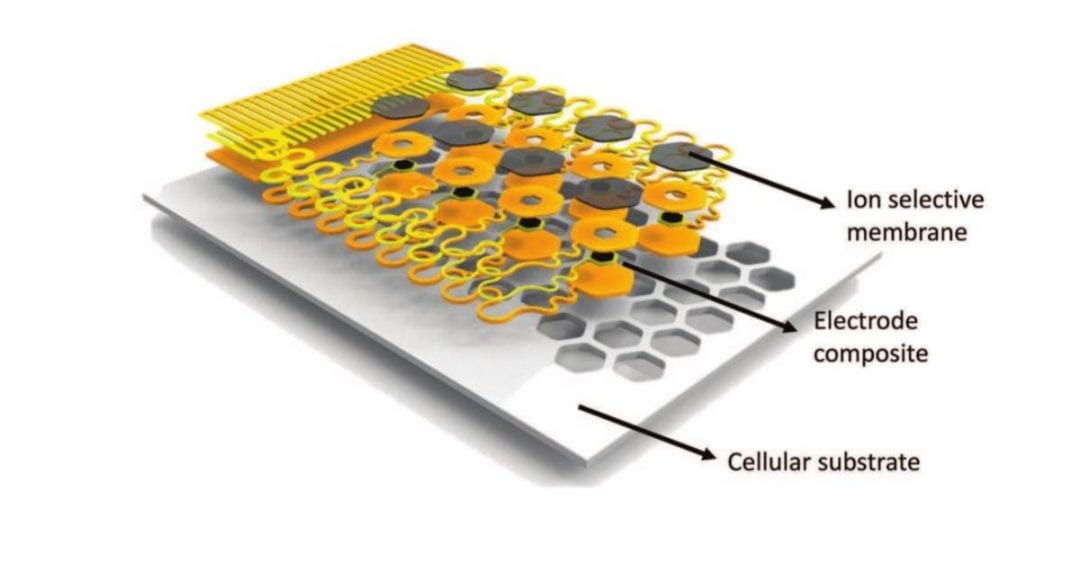
The study led by John A. Rogers and colleagues published in Advanced Functional Materials presents materials, designs and integration strategies for thin, stretchable ion sensors that mount on open, cellular substrates with well-defined geometries. Specifically, the configuration involves sensing arrays surrounded by porous areas that facilitate diffusive, convective and actively forced flows of biofluids. They provide a framework for chemical sensors capable of monitoring biomarkers in extracellular fluid, with soft physical form factors to facilitate bio-integration. Fluid permeability across the sensors minimizes accumulation artifacts induced by obstructions introduced by conventional device platforms. The envisioned advantages of this system for biological applications are in 1) capabilities for intimate and persistent contact that follows from the advanced materials integration and their soft mechanics, 2) improved accuracy in spatiotemporal mapping of target chemicals due to facilitated flow, 3) long term use of the sensor without chemical/mechanical irritation on the mounted organs.
Because the composition of extracellular fluid such as sweat and interstitial fluid contains valuable information related to biological processes, this combination of chemical sensing capabilities with stretchable cellular substrates offers potential for use across a diverse set of skin- and other organ-integrated electronics systems as an advanced form of bioelectronic sensors.