 Researchers from Washington, USA developed a process to generate hydrogen and carbon monoxide gas (sungas) from water and carbon dioxide. The gas mixture is widely used to synthesize kerosene and diesel fuels. With their process, Fang-Fang Li, Jason Lau, and Stuart Licht are able to generate sungas in an electrolysis cell powered by sunlight.
Researchers from Washington, USA developed a process to generate hydrogen and carbon monoxide gas (sungas) from water and carbon dioxide. The gas mixture is widely used to synthesize kerosene and diesel fuels. With their process, Fang-Fang Li, Jason Lau, and Stuart Licht are able to generate sungas in an electrolysis cell powered by sunlight.
Hydrogen and carbon monoxide gas is commonly synthesized via steam reforming of methane or coal. This so-called syngas is an important feedstock for fuels, such as hydrocarbons and methanol. Unfortunately, its formation involves fossil fuels and has a large carbon footprint. The reported sungas, however, is produced in an environmental friendly process from renewable resources (CO2 and H2O).
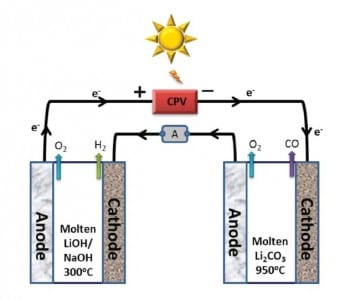
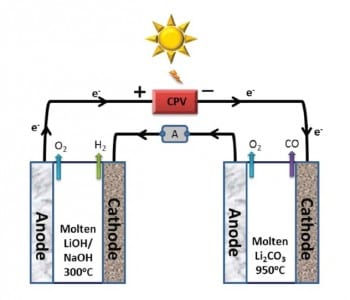
Two electrolyzers with molten Li2CO3 and LiOH/NaOH electrolytes are used for CO and H2 production, respectively. The voltage for electrolysis (2.67 V) is generated by visible sunlight in a concentrator photovoltaic (CPV). This is possible because the electrolysis potential of CO2 and H2O is reduced by heating the electrolyzers with thermal sunlight to optimized temperatures of 950 °C and 300 °C. Sungas is generated with a high Coulombic efficiency without solid carbon as a byproduct. The efficient solar thermal electrochemical process (STEM) shows 39% solar power conversion with an electrode durability of several days. Further studies will concentrate on upscaling and longer tests of the electrolysis cells.
Advanced Science is a new journal from the team behind Advanced Materials, Advanced Functional Materials, and Small. The journal is fully Open Access and is free to read now at www.advancedscience.com.