Over the past decades, stimuli-responsive polymers, which can respond to environmental stimuli such as pH-change, temperature-chang e, light, redox, and ionic strength, have attracted remarkable attention and shown great potential in various biomedical applications, including sophisticated drug/gene delivery, cell culture, tissue engineering, and biosensors. Among them, water-soluble thermoresponsive polymers exhibiting lower critical solution temperature (LCST) behavior are one of the most appealing species. Examples of LCST polymers include poly(N-isopropylacrylamide) (PNIPAAM), poly(poly(ethylene glycol) methacrylate), and elastin-like proteins.
e, light, redox, and ionic strength, have attracted remarkable attention and shown great potential in various biomedical applications, including sophisticated drug/gene delivery, cell culture, tissue engineering, and biosensors. Among them, water-soluble thermoresponsive polymers exhibiting lower critical solution temperature (LCST) behavior are one of the most appealing species. Examples of LCST polymers include poly(N-isopropylacrylamide) (PNIPAAM), poly(poly(ethylene glycol) methacrylate), and elastin-like proteins.
Since the LCST is determined by the capability of the repeat units to form hydrogen bonds with water molecules, it can be tuned both by the choice of the comonomers and by the change of the composition, according to the needs in the desired applications. Unfortunately, each copolymer requires a new polymerization process, which needs tedious organic and/or polymer synthesis and time-consuming purification. Due to the dynamic and reversible nature of noncovalent interactions, such as electrostatic interactions, π–π stacking, hydrogen bonding, and hydrophobic interactions, supramolecular functionalizations exhibit huge advantages. In sharp contast to covalent modifications, the LCST can be reversibly regulated and precisely controlled by employing various noncovalent interactions, endowing the resultant supramolecular architectures with excellent stimuli-responsiveness.
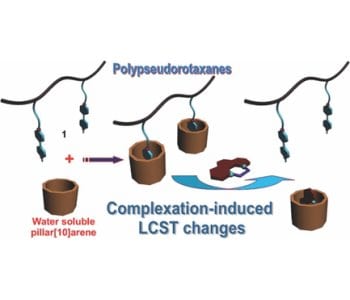
Pillar[n]arenes, a new kind of macrocyclic hosts, have gained much interest as “smart” and advanced building blocks acting as useful platforms in the fabrication of various interesting supramolecular systems, such as cyclic dimers, chemosensors, supramolecular polymers, drug delivery systems, transmembrane channels and cell glue. Yu and co-workers (Zhejiang University, Hangzhou, China) employed a water soluble pillar[10]arene (WP10) to tune the LCST behavior of a random copolymer of NIPAAM and styrene with paraquat derivative (N,N′-dialkyl-4,4′-bipyridinium) pendants (1) by taking advantage of host–guest interactions between WP10 and paraquat (PQ). 1,10-Phenanthrolinium cation (G) possessing a higher association constant with WP10 was used as a competitive guest molecule to disassociate WP10 from the host−guest complexes on the side chains of 1 caused by the size-selective complexation, which resulted in the recovery of the LCST.
This supramolecular method to tune LCST can be potentially applied in many bio-relevant fields, such as smart bioactive surfaces, enzyme recovery, protein purification and tissue engineering.