Photodynamic therapy (PDT), which is the use of a photosensitizer (PS) and a particular type of light, has been an appealing treatment strategy not only for cancer but also for various infectious diseases. However, due to non-specific and/or long-term accumulation of PSs in healthy tissues, patients going through PDT need to avoid light exposure even days after the treatment.
Activatable PSs have emerged as a solution to this problem as they are only activated at the disease site. Currently available methods to synthesize activatable PSs rely on self-quenching chemical compounds, Förster resonance energy transfer (FRET) acceptors or photo-induced electron transfer (PET) quenchers. However, these approaches suffer from constraints in molecular design and activation procedures and thus are restricted to a limited number of PSs. Therefore there is a substantial need for a general strategy that could be applied to different kind of PSs to convert them to activatable PSs for PDT.
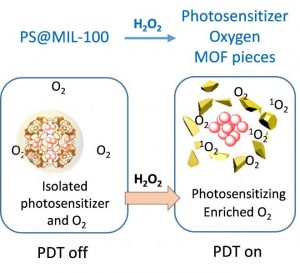
Research published in Advanced Functional Materials demonstrates an approach to transform different kinds of PSs into activatable PSs. The team led by Professor Bin Liu from National University of Singapore utilized an iron-based metal-organic framework (MOF), MIL-100 (Fe), as a nanocarrier for PSs.
This encapsulation suppressed the photosensitization until the reaction between the Fe(III) in MIL-100 (Fe) and H2O2 at the tumor site, which collapsed the framework of MIL-100 (Fe) and switched on the photosensitization of the PSs after contact with O2. O2 generated from the reaction between Fe(III) and H2O2 leads to enhanced PDT of the recovered PSs, which comes as an additional benefit of this system. The lead researcher Prof. Liu states that “We offer a simple and general strategy to yield activatable PSs by coupling MIL-100 (Fe) with existing PSs and many more new PSs to be developed.”
This simple and generic strategy enables the synthesis of activatable PSs without complex molecular engineering. Additionally, due to relieved hypoxia at the tumor site, the resulting PDT effect is enhanced. Once better control of the iron-based MOF size is achieved, this approach holds a great potential for translational research since PSs are already used in clinical setting, the as-designed nanocarriers are completely biodegradable and the degradation products have excellent body clearance.

















