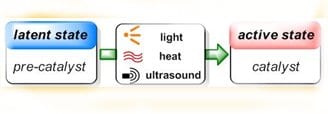
In the past decades polymers have found entrance to ever more sophisticated applications, replacing or complementing more traditional materials. This growin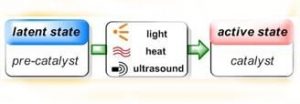 g demand has been the main driving force behind the rapid development in polymer processing technologies, which among others include the manufacturing of composite materials, coating technology, reaction-injection-molding and many more. All these technologies depend on control over the polymerization process and one of the challenges for polymer chemists has been the design of polymerization catalysts that can be “switched on” from an inactive state by application of an external trigger such as heat or light.
g demand has been the main driving force behind the rapid development in polymer processing technologies, which among others include the manufacturing of composite materials, coating technology, reaction-injection-molding and many more. All these technologies depend on control over the polymerization process and one of the challenges for polymer chemists has been the design of polymerization catalysts that can be “switched on” from an inactive state by application of an external trigger such as heat or light.
The advantages of such latent catalytic systems are numerous. Most importantly, they allow for controlling the onset of polymerization exactly where and when it is desired, but they also provide enhanced production safety and simplify the technological setup as the problematic in-situ addition of (highly reactive) chemicals and mixing problems are avoided. Latent catalysts can be stored together with the monomers without premature reaction occurring, thus enabling the use of single-component formulations that are ready to polymerize, simply by application of the appropriate stimulus.
Appealing as these beneficial aspects of latent catalysis are, realization remains still challenging in many cases. A fast and quantitative activation step is desired, which means that the transition from perfect latency to high activity has to be swift, and yet the pre-catalysts have to be stable enough to remain inert under storage conditions in a chemical environment that contains fillers, stabilizers, solvents and other additives. Furthermore, the processing conditions themselves are highly versatile, ranging from large polymer parts to ultra-thin films, from polymerizations under protective gas atmosphere to outdoor applications, from light-weight construction to dental fillings. This imposes severe requirements on the latent catalysts, and it is clear that good performance can only be expected from a clever design of these.
Naumann and Buchmeiser now review established as well as just emerging latent polymerization systems, focusing on the multiple chemical venues that can be taken to achieve latent behavior. It is shown that matching latent polymerization techniques exist for the individual polymer processing methods. Special emphasis is put on protected N-heterocyclic carbenes, a relatively new class of latent catalysts. Mechanistic considerations and future challenges are discussed and the advantageous application of latent catalysis is demonstrated, including the preparation of polyurethanes, polyamides, polyesters, polyacrylates, epoxy resins and metathesis-derived polymers employing (UV-)light, heat, mechanical force or electrochemical potentials as external triggers. The presented examples cover ionic and radical as well as metathesis- and polyaddition-based mechanisms.