Since water is an abundant resource and hydrogen can directly be used as carbon-free chemical fuels, photochemical water splitting is an elegant way to solve both energy and environmental issues. A major limitation of efficient photochemical water splitting lies in finding an highly active photooxidation catalyst. Many traditional photocatalysts suffer from a large bandgap energy, which restricts their light absorption to the UV region, and the severe recombination of photoinduced charges, which limits the overall efficiency of the device.
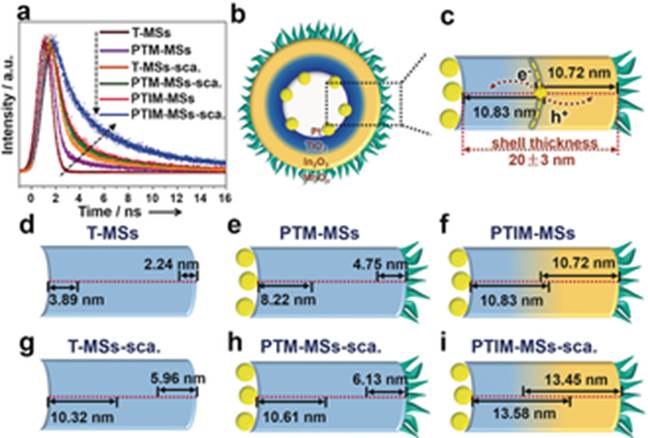
Prof. Jinlong Gong and his colleagues at Tianjin University have recently developed mesoporous hollow spheres with the composition Pt@TiO2@In2O3@MnOx. They can absorb both UV and visible light. Owing to the presence of thin heterojunctions and spatially separated cocatalysts, charge recombination is also efficiently reduced in both the bulk phase and the surface/subsurface. When used in photooxidation reactions, these mesoporous hollow spheres exhibited high activity for both water oxidation and selective benzyl alcohol oxidation. The O2 evolution rate (466.6 μmol g−1 h−1) was higher than for conventional TiO2-based catalysts without decoration (typically 67–180 μmol g−1 h−1). For benzyl alcohol oxidation, the apparent quantum efficiency reached 49.2 % and 24.2 % under UV (254 nm) and visible (435 nm) irradiation, which is also higher than that of bare TiO2 (typically 17 %–39 % under UV light and 4.3 %–20 % under visible light).
Considering the enhanced light adsorption, charge separation and surface reaction, the authors believe that such a novel structure can also provide inspiration for other photocatalytic systems, for example, for light-assisted CO2 reduction.