Challenges underlying the fluorescence sensing of biologically important molecules are the detection and differentiation of a target molecule from its structural analogues. As an example, pyridine nucleotide cofactors (NAD+, NADH, NADP+, and NADPH) can be raised. The NADPH produced in pentose phosphate pathway plays an essential role in balancing the redox state of living cells. To probe NADPH, one needs to differentiate it from the other three structural analogues.
Challenges underlying the fluorescence sensing of biologically important molecules are the detection and differentiation of a target molecule from its structural analogues. As an example, pyridine nucleotide cofactors (NAD+, NADH, NADP+, and NADPH) can be raised. The NADPH produced in pentose phosphate pathway plays an essential role in balancing the redox state of living cells. To probe NADPH, one needs to differentiate it from the other three structural analogues.
Although precise differentiation of the cofactors based on their net-charges derived from the difference in the number of phosphate groups is inevitably required, it is difficult to discriminate a concerned target from its structural analogue by conventional fluorescence sensors because of their 1:1 binding mode between the receptor and the phosphates.
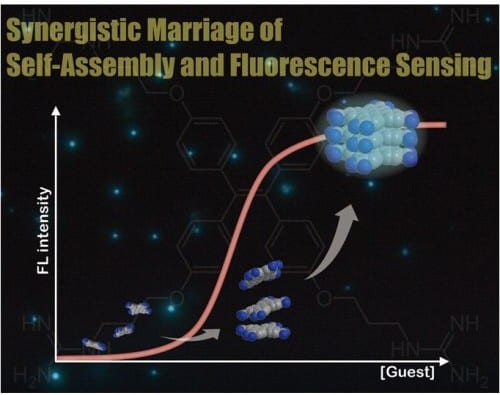
The principle of molecular self-assembly is expected to afford a new dimension to the field of fluorescence sensing, because subtle difference in the molecular structure can be amplified into the resulting self-assembled structures as an output according to the molecular information encoded. To utilize the self-assembly as a practical tool, the group around Seiji Shinkai (Kyushu University, Japan) now focuses on an aggregation-induced emission (AIE) dye, which is endowed to show the “turn-on” fluorescence switching in response to self-assembly formation. They demonstrate that synergistic marriage of fluorescence sensing and molecular self-assembly makes selective recognition of NADPH possible by a steep turn-on fluorescence response accompanied by the high signal-to-background contrast. Such a differentiation based on molecular self-assembly has never been realized by conventional fluorescence sensing based on a 1:1 binding system via molecular recognition.