The plastics industry is one of the fastest growing in the world. The global plastics demand in 2015 was over 300 million tons, and this number is growing steadily each year. The low cost of production and the ease with which they are processed combine to make plastics usable in a wide variety of applications, from food packaging to insulation to clothing.

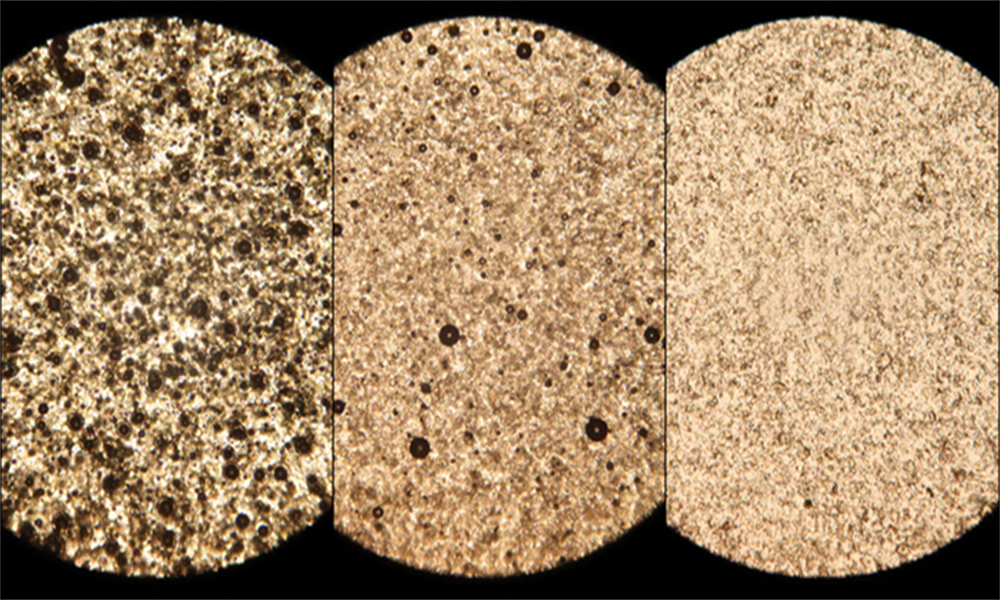
This image shows the TA-based epoxy resin when mixed at an elevated temperature of 150°C. From left to right, the optical microscopy images show the resin after 0, 20, and 40 minutes of mixing. The progressive mixing and hardening of the epoxy resin can be seen in the lightening color and decreased number of TA clumps.
Unfortunately, the source of most commercial plastics is petroleum, and almost half of the total amount of plastics is only used short-term. As Matthew Korey and colleagues point out in their study, published in the Journal of Polymer Science Part A: Polymer Chemistry, this means “that producers are using nonrenewable resources to make a product that will be used once or twice and then put into waste.”
The team also points out another drawback to those plastics specifically utilized in the adhesives and coating industries. These plastics are made up of thermoset polymers, which in turn contain epoxy-based polymers, known for their mechanical strength, adhesion to many substrates, and excellent thermal, electrical, and chemical resistance properties. The main concerns that currently face the epoxy thermoset industry are: one, that the precursors come from nonrenewable petroleum, and two, that the precursors are toxic to humans and the environment.
To this end, Korey and team focused on an alternative source for epoxy hardening agents: tannic acid (TA). Tannic acid is a naturally occurring compound found in nuts, seeds, and tree bark that is labeled safe by the FDA. The cost of TA is comparable to the chemical hardeners currently in use, but it has the advantage of being renewable and nontoxic. This study demonstrated that TA was able to react and form an epoxy polymer with high thermal stability analogous to several epoxy thermosets that are currently commercially available.
There was a current limitation found by this work, namely, the high temperature required for the reaction to ensure the TA based epoxy would have sufficient mechanical and thermal properties. Adjustments to future methods could allow for the cross-linking reaction to occur at lower temperatures. Korey and colleagues hope that their work will encourage the further study and ultimate use of TA as a more health and environmentally friendly epoxy hardening agent.