Many devices that we use regularly in our lives are battery-powered. Mobile phones, laptops, e-readers, game console controllers, cameras and power tools are all battery-powered, and even mostly powered by rechargeable batteries. While large rechargeable batteries power electric cars, and store energy produced by solar panels. It goes without saying that rechargeable batteries play a significant role in modern life.
Batteries-based on lithium (Li) are the most common rechargeable battery available and in use. Each year new research aims to improve the performance of Li–ion batteries. Lithium–air (Li–O2) batteries are promising candidates for next-generation storage devices.
In conventional lithium–air batteries, limitations in mass transport exist, most specifically related the cathode. Active sites on the cathode can be under-utilized, which can lead to capacity loss. To improve performance, alternatives have been researched, including using various electrolytes and a variety of cathode materials. However, few researchers have focused their attention on fundamentally modify the architecture of the cathode to resolve the transport issues.
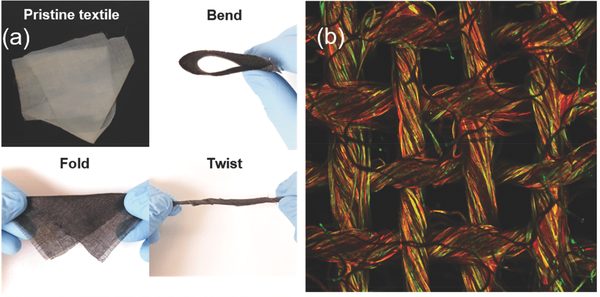
The flexibility and morphology of the textile-based air cathode. More information here.
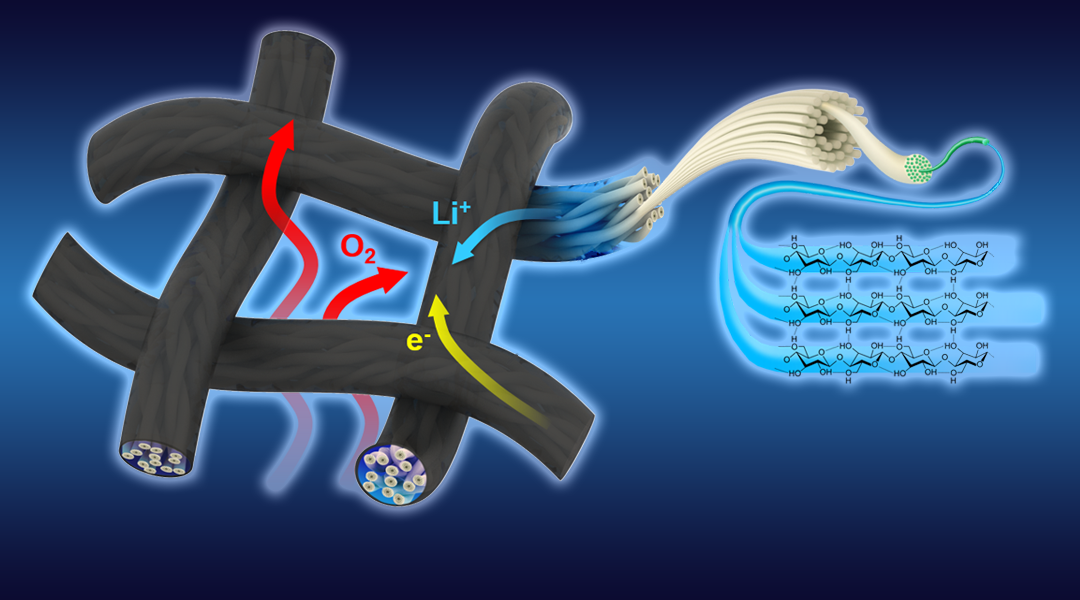
In their research, Professor Liangbing Hu, Dr Jun Lu and co-workers, developed a novel flexible textile-based cathode with a unique triple-phased structure for improved non-competitive transport properties.
The woven textile-based cathode exhibits an open structure that enables separate pathways for electrolyte and oxygen transport. The mechanisms by which this works is that the electrolyte transfers along the fibers of the textile, while the oxygen can diffuse through the woven mesh pores. What seems like a simple solution provides a dramatic new direction for the fabrication and design of Li–air cathodes. This design moves beyond trying to increase surface area and controlling pore size.
Dr. Liangbing Hu highlighted technologies that this research could impact saying, “The application of textile-based triple-phase structures is not limited to Li–air batteries. Fuel cells, electrocatalytic or photocatalytic water spitting, and redox flow batteries can also take advantage of interlayers with individual pathways for both the liquid phase and the gas phase.”