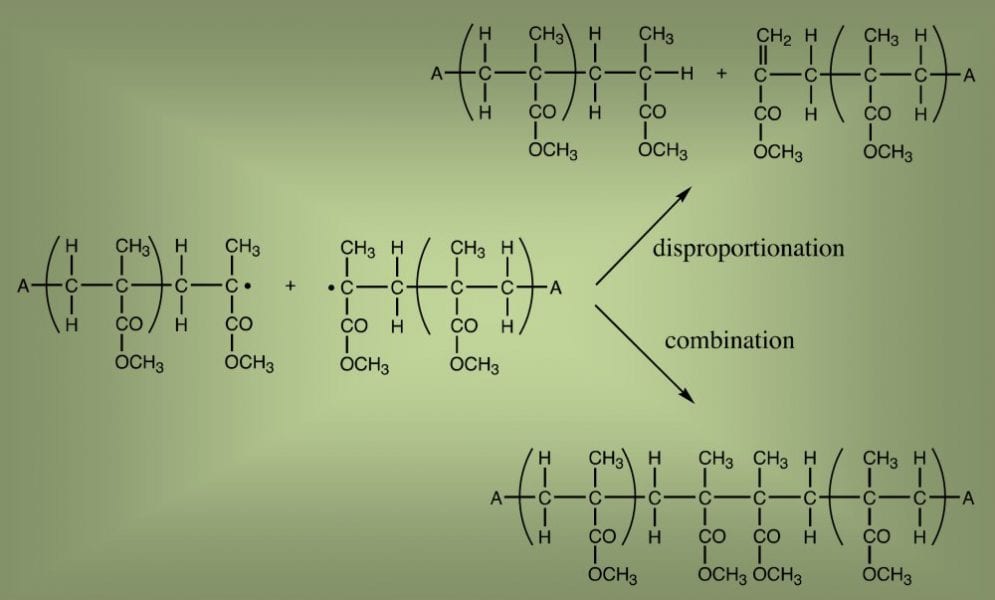
Synthetic polymers are macromolecules but they are nevertheless finite in size. In radical polymerization this is because there are reactions that eventually stop the growth of a forming polymer molecule. The two such reactions that may always occur are combination and disproportionation. This is because they are intrinsic to radical reactivity and do not require any other reactants.
Combination is the marriage of two macroradicals, from which they emerge as the one conjoined entity of combined size. Disproportionation, on the other hand, involves a short dalliance, after which the two reactants go their separate ways, but changed into a non-reactive state. Because these reactions therefore result in products of different size, as illustrated in the graphic below, it is important to know the relative extent to which each occurs.
Historically, this has been surprisingly difficult to determine with accuracy. However, the advent of large-molecule mass spectrometry, with its capacity for precise identification of polymer nature and size, offers the chance to remedy this situation.
In their present work, Gregory T. Russell et al. (University of Canterbury, New Zealand) road-test their recently proposed method for determining the balance between combination and disproportionation via mass spectrometry. They show that the method is robust across a variety of conditions, and emphasize that it is elegantly reliant on the broader kinetic scheme for radical polymerization. Therefore they establish that the method is ripe for wider exploitation.