Peptides have great potential as self-assembly building blocks on account of their modular nature and protein-like functionalities. Weak interactions contributing to molecular self-assembly are easily influenced by environmental conditions, enabling self-assembling structures to exhibit interesting functionalities in response to different external stimuli, including temperature, pH, ionic strength, and enzymatic reactions.
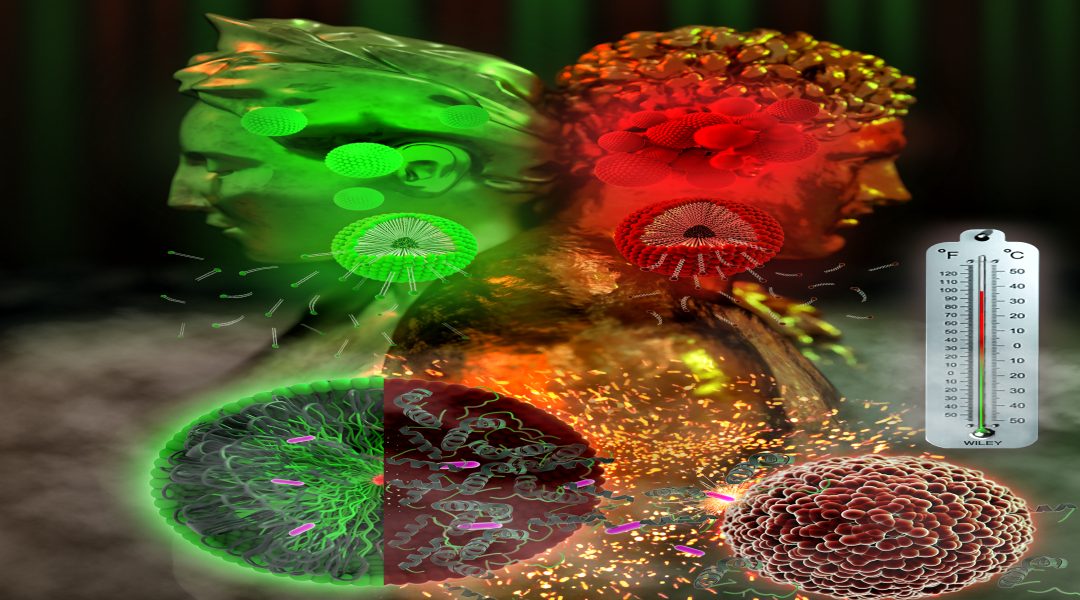
In particular, elastin-like peptides (ELPs) can impart thermoresponsiveness to self-assembling structures because ELPs form insoluble coacervates and undergo a conformation transition from random coils (hydrophilic state) to β-spirals (hydrophobic state) when the temperature surpasses a certain value. For example, ELPs that aggregate in response to the temperature increase surrounding cancer cells (compared to that of normal cells or outside the body) can be used in cancer therapy. However, introduction of ELPs into peptide self-assembly systems is limited by their large size.
Yong-beom Lim and colleagues from the Department of Materials Science and Engineering, Yonsei University, design a facile strategy to utilize the thermoresponsiveness of ELPs within a temperature range suited for biological applications.
The miniaturized ELPs (MELPs) designed by the researchers contain less than 23 amino acids and exhibit rapid coacervation and retarded disaggregation behavior in response to heating and cooling, respectively. These synthetic MELPs are much smaller than conventional ELPs, which contain several tens to hundreds of amino acids.
An MELP platform grafted with an α-helical guest peptide was shown to be effective for drug delivery in response to temperature changes and could have great potential in the development of temperature-responsive smart materials.
The authors state, “This synthetic method can exploit the high degree of freedom in small peptide design and enable MELPs to have diverse sophisticated structures and to be conjugated with other functional materials.”
To find out more, read the article here.
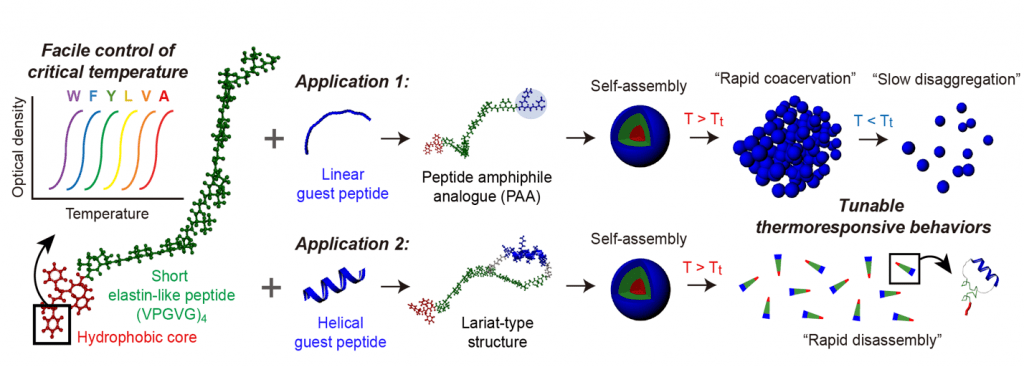
MELP platform with tunable thermoresponsiveness.