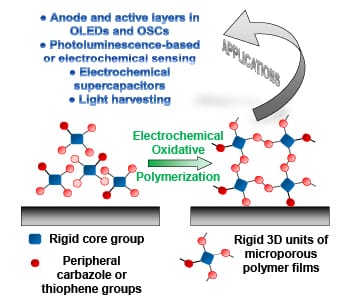
Due to their high porosity and intrinsic surface area, microporous polymer networks show great application potential in gas storage and separation, heterogeneous catalysis, and photo-luminescence-based sensing. Since these network consist of highly cross-linked organic structures, they are however insoluble and cannot be processed into thin films by traditional methods. One way to achieve these thin films is electrochemical polymerization, where the film is generated directly on the working electrode. In their recent trend article, Alex Palma-Cando and Ullrich Scherf discuss the synthesis, properties, and applications of these electrochemically generated thin films of microporous polymer networks.
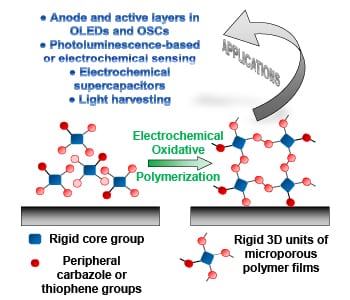 The monomers for these thin films are rigid 3D structures that often carry multiple carbazole and thiophene moieties on their edges for convenient electropolymerization, which is conducted in a three-electrode cell. These monomers are oxidized at the working electrode, and in radical coupling reactions, form oligomers and then polymers.
The monomers for these thin films are rigid 3D structures that often carry multiple carbazole and thiophene moieties on their edges for convenient electropolymerization, which is conducted in a three-electrode cell. These monomers are oxidized at the working electrode, and in radical coupling reactions, form oligomers and then polymers.
Thin films produced by this approach are generally microporous with high BET surface areas up to 2000 m2g-1 and low average surface roughness. A smooth surface of the films is a key prerequisite for application in organic electronic devices (e.g., OLEDs and OSCs), and was for long time difficult to achieve with electrogenerated conducting polymer films.
Many potential applications for this type of material have been presented during the last three years, especially for the use in photo luminescence and electrochemical sensing, electrochemical supercapacitors, light-harvesting antennae, OLEDs, and OSCs. For the future, many more application examples can be expected.