 Radical-mediated thiol-ene chemistry is a prominent photopolymerization technique for the synthesis of functional polymers and advanced polymer networks. The reaction can be applied to numerous thiol and alkene monomers and benefits from mild reaction conditions, high efficiency, insensitivity to oxygen, high yields, and negligible formation of side products. In particular, thiol-ene photopolymers have gained increased attention in the design of functional materials for tissue engineering, nano-imprinting, shape memory or optical and microfluidic devices.
Radical-mediated thiol-ene chemistry is a prominent photopolymerization technique for the synthesis of functional polymers and advanced polymer networks. The reaction can be applied to numerous thiol and alkene monomers and benefits from mild reaction conditions, high efficiency, insensitivity to oxygen, high yields, and negligible formation of side products. In particular, thiol-ene photopolymers have gained increased attention in the design of functional materials for tissue engineering, nano-imprinting, shape memory or optical and microfluidic devices.
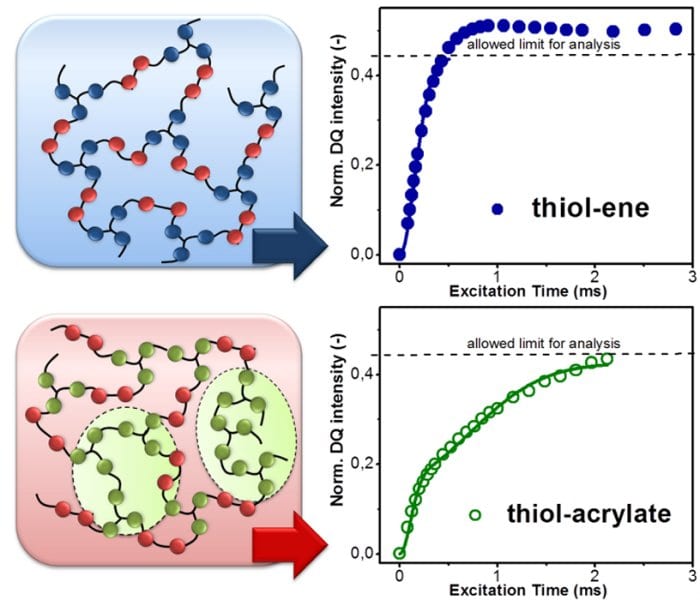
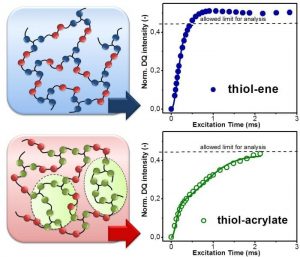
Recently, a team led by Sandra Schlögl (Polymer Competence Center Leoben GmbH, Austria) provided a deeper insight into the macromolecular structure of thiol-ene and thiol-acrylate photopolymers. They employed low field double quantum NMR (DQ NMR) techniques to study network properties and chain dynamics of photopolymers in dependence on the chemical structure of both thiol as well as alkene monomer. The network homogeneity plays an important role for the macroscopic properties of the photopolymers as it governs polymerization shrinkage stress, mechanical performance and glass transition temperature.
The authors showed that thiol-ene photopolymers provide a uniform network structure due to the well-defined step-growth mechanism of the photopolymerization between thiol and alkene monomers. In contrast, photopolymerization between thiol and acrylate monomers yields heterogeneous networks with a vastly different mobility resulting from the intertwined network formed by simultaneous chain growth (homopolymerization of acrylate monomers) and step growth polymerization.
By going from the molecular structure of the monomers over the dynamics of the polymer networks to the final mechanical properties, it was demonstrated that the yield of homopolymerization contributes to the heterogeneity of thiol-ene networks, which also clearly influences the corresponding thermo-mechanical properties such as tensile properties and glass transition temperature.