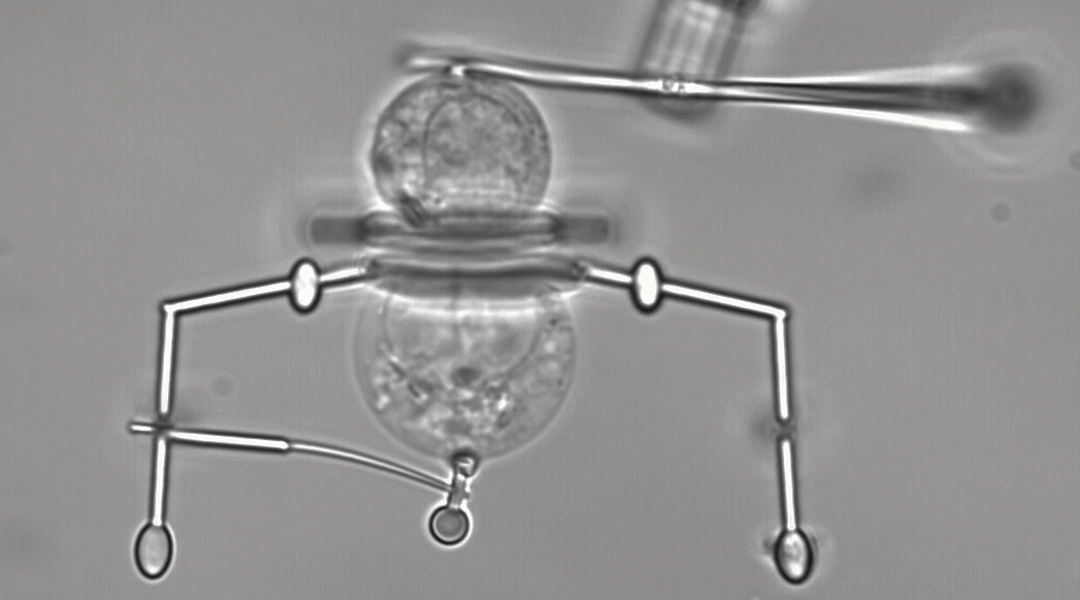
Scientists have created a family of laser-operated microrobots that can handle one of life’s tiniest units: the cell.
The nanoscale robots can grip, move, and rotate single cells, which are 30 to 40 micrometers large. The precision these microrobots offer for imaging single cells and their ability to manipulate interactions between them magnify researchers’ ability to answer fundamental questions.
Lóránd Kelemen, a biophysicist at the HUN-REN Biological Research Centre in Szeged, Hungary, worked with colleagues in Hungary and Slovakia to develop a range of microrobotic tools that work in combination with optical tweezers, a decades-old technique that scientists use to trap microscopic objects in focused laser beams.
“If a small object gets into the focus of the beam, the beam gets refracted by the object and it changes direction,” explained Kelemen. According to the physics of light, the change in direction means there is a change in the momentum of the light as well.
“But momentum change means that a force was applied on the beam that caused this change and since it is the small object that refracted the beam, the small object exerted force on the beam,” continued Kelemen. For every action there is an equal and opposite reaction, meaning the light beam exerts force back on the small object keeping it in place.
Making optical tweezers a little more gentle
Using optical tweezers on their own to study single cells is not perfect because trapping the cell itself in the beam heats up and damages some of the cellular machinery.
The stability and force generated by the tweezers are also weak due to the size and shape of the cell in relation to the beam itself and the water it’s suspended in.
To remedy this, in the past scientists have adhered small microbeads or structures to cells that act like handles the optical tweezers can hold onto. Unfortunately, these too damage the cell, and once added, cannot be removed.
“It is similar to using an adhesive tape to mount a cotton ball to a spatula,” Kelemen explained. “You can grab the spatula to move the ball around without squeezing it, but when you want to remove it you tear the ball apart.”
Often, these attachments also require changes to the cells’ environment, like pH or adding other chemicals to complete the bonding. The new microtools developed in the current study avoid this and are small and flexible enough to gently grip cells while themselves being gripped by the beam of the optical tweezer.
The microrobots required another set of lasers to build. A process called two-photon polymerization uses light to polymerize, or harden, a polymer only where the light touches the material.
“We focus the light down to a fraction of a micrometer and the polymer hardens only at that tiny spot,” said Kelemen. This allowed them to precisely craft the tools to nanometer size specifications.
The microrobots do well in tests
The challenge was making the tools thin and flexible enough to grip the cells while being gripped by the beam themselves. “We had to be very precise because if an elastic element in our microstructure is 400 nm thick it can be just bent by the optical tweezers, but if it’s 600 nm thick it cannot,” he explained.
In tests, the team showed they could use the beam from the optical tweezers to deform one part of the microrobots, opening them up to grab the cell, for example. Once in their grip, the elasticity of the microrobot keeps the cell in place, freeing up the beam to trap a stiffer part of the tool and manipulate it, much like the cotton ball and spatula example above.
The team designed three tools that allowed the researchers to transfer single cells from one place to another, precisely rotate a cell for microscopy imaging, or to grab two cells and press them together to study their reactions.
“It was a long development process, with lots of surprises on the way,” said Kelemen. However, the tools work as intended and provide a non-destructive way to manipulate and image single cells in a natural environment.
As optical tweezers are still specialized, Kelemen doesn’t expect every lab to have the capacity to use their new microtools but those that do have the equipment can modify and optimize them for new tasks.
“Sometimes the elastic rods need to be longer or a structural part a little shorter or to come in a different angle,” Kelemen said. Now, with their blueprint and the growing availability of two-photon polymerization systems, “laboratories who can afford them can in principle produce such structures.”
Reference: Lóránd Kelemen, et al., Optically Actuated Soft Microrobot Family for Single-Cell Manipulation, Advanced Materials (2024). DOI: 10.1002/adma.202401115